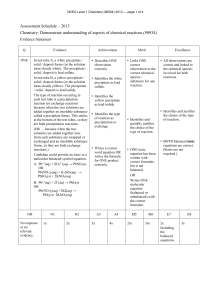
Chemistry - University of Kashmir
... structural diagnosis. Prepration, reactions, structure, bonding of transition metal Nitrosyls, and Dinitrogen and Dioxygen complexes of transition metals. Tertiary phosphine as ligand. Organometallic Compounds Definition, nomenclature and classification of organometallic compounds. Effective atomic ...
... structural diagnosis. Prepration, reactions, structure, bonding of transition metal Nitrosyls, and Dinitrogen and Dioxygen complexes of transition metals. Tertiary phosphine as ligand. Organometallic Compounds Definition, nomenclature and classification of organometallic compounds. Effective atomic ...
Thermodynamics - TCD Maths home
... Definition: (Equilibrium State, State Variables) An equilibrium state is one in which all the bulk physical properties of the system are uniform throughout the system and do not change with time. An equilibrium state is specified by two independent variables known as state variables. Definition: (Th ...
... Definition: (Equilibrium State, State Variables) An equilibrium state is one in which all the bulk physical properties of the system are uniform throughout the system and do not change with time. An equilibrium state is specified by two independent variables known as state variables. Definition: (Th ...
File
... atomic emission spectra; Bohr model of the hydrogen atom including explanation of H line spectrum and orbits, electron cloud and the probability model, wave/particle duality of electrons revisited, relate electron configurations of atoms to the Bohr and electron cloud models, describe the concepts o ...
... atomic emission spectra; Bohr model of the hydrogen atom including explanation of H line spectrum and orbits, electron cloud and the probability model, wave/particle duality of electrons revisited, relate electron configurations of atoms to the Bohr and electron cloud models, describe the concepts o ...
Syllabus - Chemistry
... Valence bond theory- Energy changes taking place during the formation of diatomic molecules; factors affecting the combined wave function. Bent's rule and energetics of hybridization.Resonance: Conditions, Resonance energy and examples of some inorganic molecules/ions. Molecular orbital theory- Vari ...
... Valence bond theory- Energy changes taking place during the formation of diatomic molecules; factors affecting the combined wave function. Bent's rule and energetics of hybridization.Resonance: Conditions, Resonance energy and examples of some inorganic molecules/ions. Molecular orbital theory- Vari ...
PPT - mvhs-fuhsd.org
... increased entropy (more space for random movement) 4. If the pressure on a container of gas is increased, then the entropy decreases (due to restricted movement of particles) • Arrange ice, water and water vapor in order of decreasing entropy. ...
... increased entropy (more space for random movement) 4. If the pressure on a container of gas is increased, then the entropy decreases (due to restricted movement of particles) • Arrange ice, water and water vapor in order of decreasing entropy. ...
PHYSICAL SETTING CHEMISTRY
... questions on this separate answer sheet. Record your answers for the questions in Part B–2 and Part C in your separate answer booklet. Be sure to fill in the heading on the front of your answer booklet. All answers in your answer booklet should be written in pen, except for graphs and drawings, whic ...
... questions on this separate answer sheet. Record your answers for the questions in Part B–2 and Part C in your separate answer booklet. Be sure to fill in the heading on the front of your answer booklet. All answers in your answer booklet should be written in pen, except for graphs and drawings, whic ...
82KB - NZQA
... solid, CaCO3, is strongly heated it releases a colourless gas, carbon dioxide, CO2, and forms another white solid calcium oxide, CaO. CaCO3(s) → CaO(s) + CO2(g) When white calcium hydroxide solid, Ca(OH)2, is strongly heated it releases a colourless gas, water, H2O, and also forms the white solid ca ...
... solid, CaCO3, is strongly heated it releases a colourless gas, carbon dioxide, CO2, and forms another white solid calcium oxide, CaO. CaCO3(s) → CaO(s) + CO2(g) When white calcium hydroxide solid, Ca(OH)2, is strongly heated it releases a colourless gas, water, H2O, and also forms the white solid ca ...
Final Exam - Seattle Central College
... – Recognize that London forces increase with more electrons—use size to determine relative number of electrons for different molecules. – Know the terms: evaporation, boiling point, vapor pressure, volatile, nonvolatile – Recognize how IMF’s influence vapor pressure and boiling point. – Given differ ...
... – Recognize that London forces increase with more electrons—use size to determine relative number of electrons for different molecules. – Know the terms: evaporation, boiling point, vapor pressure, volatile, nonvolatile – Recognize how IMF’s influence vapor pressure and boiling point. – Given differ ...
The Chemical Basis of Life
... Isotopes of an element – Different forms of an element with the same atomic number but with different mass numbers – The atoms of some isotopes are stable – Other isotopes are radioactive, having unstable atoms that spontaneously break apart (decay) to form other atoms – When radioactive atoms decay ...
... Isotopes of an element – Different forms of an element with the same atomic number but with different mass numbers – The atoms of some isotopes are stable – Other isotopes are radioactive, having unstable atoms that spontaneously break apart (decay) to form other atoms – When radioactive atoms decay ...
Chapter 6 Thermal Energy
... through pipes outside the house. There, the coolant absorbs heat from the outside air. The coolant is then compressed and pumped back inside the house, where it releases heat. ...
... through pipes outside the house. There, the coolant absorbs heat from the outside air. The coolant is then compressed and pumped back inside the house, where it releases heat. ...
Equation Chapter 1 Section 1 Tips for Studying: Take responsibility
... 2. The reservoir stores water at a higher level than the generator below the dam, so the water has gravitational potential energy due to its higher position. 3. Water is the released into the penstock. As it flows down the penstock it loses gravitational potential energy but gains kinetic energy as ...
... 2. The reservoir stores water at a higher level than the generator below the dam, so the water has gravitational potential energy due to its higher position. 3. Water is the released into the penstock. As it flows down the penstock it loses gravitational potential energy but gains kinetic energy as ...
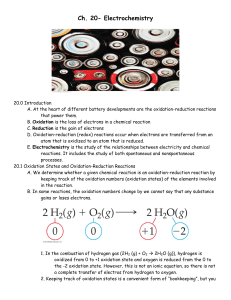
Ch. 20- Electrochemistry
... convenient to consider them as separate processes. b. Equations that show either oxidation or reduction along are called halfreactions. c. In the overall redox reaction, the number of electrons lost in the oxidation half-reaction must equal the number of electrons gained in the reduction halfreactio ...
... convenient to consider them as separate processes. b. Equations that show either oxidation or reduction along are called halfreactions. c. In the overall redox reaction, the number of electrons lost in the oxidation half-reaction must equal the number of electrons gained in the reduction halfreactio ...
Periodic Table, Bonding, Reactions, and Moles
... 13. Identify the two types of bonds in the compound sodium stearate. Base your answers to questions 14 through 16 on the information below. The Solvay process is a multistep industrial process used to produce washing soda, Na2CO3 (s). In the last step of the Solvay process, NaHCO3 (s) is heated to 3 ...
... 13. Identify the two types of bonds in the compound sodium stearate. Base your answers to questions 14 through 16 on the information below. The Solvay process is a multistep industrial process used to produce washing soda, Na2CO3 (s). In the last step of the Solvay process, NaHCO3 (s) is heated to 3 ...
Momentum Heat Mass Transfer
... Such a new law is the equation of internal energy balance which represents the first law of thermodynamics stating, that the internal energy increase is determined by the heat delivery by conduction and by the mechanical work. This statement can be expressed in form of a general transport equation f ...
... Such a new law is the equation of internal energy balance which represents the first law of thermodynamics stating, that the internal energy increase is determined by the heat delivery by conduction and by the mechanical work. This statement can be expressed in form of a general transport equation f ...
Solving Equilibrium Problems
... Write the equilibrium expression List or calculate initial concentrations (or pressures) with information given If there are products present initially, calculate Q to make sure system is not already at equilibrium ...
... Write the equilibrium expression List or calculate initial concentrations (or pressures) with information given If there are products present initially, calculate Q to make sure system is not already at equilibrium ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.