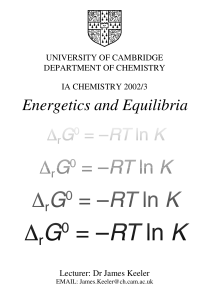Keq Assignment
... g) calcium ions and phosphate ions come out of solution to produce solid calcium phosphate. ...
... g) calcium ions and phosphate ions come out of solution to produce solid calcium phosphate. ...
Chapter Two The Thermodynamic Laws
... "Heat cannot of itself pass from a colder to a hotter body." This statement implies an inequality of the heat transfer between a hot body and a cold body. Heat transfer from a hot body to a cold body can spontaneously occur. However, heat transfer in the reversed direction can not happen without the ...
... "Heat cannot of itself pass from a colder to a hotter body." This statement implies an inequality of the heat transfer between a hot body and a cold body. Heat transfer from a hot body to a cold body can spontaneously occur. However, heat transfer in the reversed direction can not happen without the ...
MSDS - Dudley Chemical Corporation
... representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this. Individuals receiving the information must exercise their independent judgment in determining its appro ...
... representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this. Individuals receiving the information must exercise their independent judgment in determining its appro ...
Physical Chemistry
... 3 hous long Chapter 7. Phase equillibriums: binary II and ternary systems. S-L equillibrium: Solubility of a solid in a liquid. Cooling curves: Phase diagrams, eutectic point. L-L equillibrium: phase diagrams. Ternary systems: triangle plots. 3 hours long Chapter 8. Colligative properties. Decrease ...
... 3 hous long Chapter 7. Phase equillibriums: binary II and ternary systems. S-L equillibrium: Solubility of a solid in a liquid. Cooling curves: Phase diagrams, eutectic point. L-L equillibrium: phase diagrams. Ternary systems: triangle plots. 3 hours long Chapter 8. Colligative properties. Decrease ...
Final Review
... 8. From your knowledge of intermolecular forces, arrange the following in order of increasing surface tension (least to most): Water, hexane, ethanol, ethanal 9. Describe how the intermolecular forces in water allow for each of the following properties of water: a. low vapor pressure c. solid H2O is ...
... 8. From your knowledge of intermolecular forces, arrange the following in order of increasing surface tension (least to most): Water, hexane, ethanol, ethanal 9. Describe how the intermolecular forces in water allow for each of the following properties of water: a. low vapor pressure c. solid H2O is ...
UNIT I PART B 1). (i). A spherical balloon of diameter
... the system, they will get neutralized to attain the condition of equilibrium. Two systems are said to be in mechanical equilibrium with each other when their pressures are same. 2) Chemical equilibrium: The system is said to be in chemical equilibrium when there are no chemical reactions going on w ...
... the system, they will get neutralized to attain the condition of equilibrium. Two systems are said to be in mechanical equilibrium with each other when their pressures are same. 2) Chemical equilibrium: The system is said to be in chemical equilibrium when there are no chemical reactions going on w ...
C. Adding acid shifts the equilibrium to the right
... the element. Isotopes of an element have different mass numbers because they have different numbers of neutrons, but they all have the same atomic number. Electron configurations represent the way electrons are arranged in atoms. Electrons enter the lowest energy first. At most there can be only 2 e ...
... the element. Isotopes of an element have different mass numbers because they have different numbers of neutrons, but they all have the same atomic number. Electron configurations represent the way electrons are arranged in atoms. Electrons enter the lowest energy first. At most there can be only 2 e ...
worksheet Ka Kb buffers Ksp
... Consider the following rate law expression: rate = k[A]2[B]. Which of the following is not true about the reaction having this expression? a. The reaction is first order in B. b. The reaction is overall third order. c. The reaction is second order in A. d. Doubling the concentration of A doubles the ...
... Consider the following rate law expression: rate = k[A]2[B]. Which of the following is not true about the reaction having this expression? a. The reaction is first order in B. b. The reaction is overall third order. c. The reaction is second order in A. d. Doubling the concentration of A doubles the ...
Final Exam Study Guide Chapters 1-12
... ____ 55. In metallic bonds, the mobile electrons surrounding the positive ions are called a(n) a. Lewis structure. c. electron cloud. b. electron sea. d. dipole. ____ 56. To appear shiny, a material must be able to a. form crystals. b. absorb and re-emit light of many wavelengths. c. absorb light an ...
... ____ 55. In metallic bonds, the mobile electrons surrounding the positive ions are called a(n) a. Lewis structure. c. electron cloud. b. electron sea. d. dipole. ____ 56. To appear shiny, a material must be able to a. form crystals. b. absorb and re-emit light of many wavelengths. c. absorb light an ...
Chapter 17 - Cengage Learning
... The collision model says that in order for molecules to react with each other, they must first collide. Increases in the temperature and concentration of reactants bring about more collisions, and the rate of reaction increases. The collision model explains many observations about reactions. Not all ...
... The collision model says that in order for molecules to react with each other, they must first collide. Increases in the temperature and concentration of reactants bring about more collisions, and the rate of reaction increases. The collision model explains many observations about reactions. Not all ...
Chapter 15: Thermal Properties of Matter
... • Relate the macroscopic properties to the microscopic properties • Gain an understanding of the thermal properties of matter • Consider various phases of matter: gas, liquid, and solid and conditions under which they occur ...
... • Relate the macroscopic properties to the microscopic properties • Gain an understanding of the thermal properties of matter • Consider various phases of matter: gas, liquid, and solid and conditions under which they occur ...
Document
... This is why heat pumps are much better than electric heaters. Instead of 1000 J of work going to 1000 J of heat we have 1000 J of work producing 7500 J of heat. ...
... This is why heat pumps are much better than electric heaters. Instead of 1000 J of work going to 1000 J of heat we have 1000 J of work producing 7500 J of heat. ...
2017 Chemistry Exam Review Compounds and Reactions 1. Know
... reaction. In each graph, show the reactants and products, indicate where bonds are broken and made and label the change of enthalpy (H), including whether it is positive or negative. 33. Describe a calorimeter and how it works. 34. Describe how we determined experimentally the heat of combustion fo ...
... reaction. In each graph, show the reactants and products, indicate where bonds are broken and made and label the change of enthalpy (H), including whether it is positive or negative. 33. Describe a calorimeter and how it works. 34. Describe how we determined experimentally the heat of combustion fo ...
A2 Chemistry key word list
... products that allows the indirect determination of an enthalpy change from other known enthalpy changes using Hess’s law. ...
... products that allows the indirect determination of an enthalpy change from other known enthalpy changes using Hess’s law. ...
Classwork – Nature, Properties, and Classification of Matter
... Classwork – Chemical Quantities (Stoichiometry) 1. Using sandwich making as an analogy to chemical reactions, show the balanced equation that requires 2 pieces of bread, 3 slices of meat and on slice of cheese to make 1 sandwich. 2. Using the ratios for the above process (reaction), show the balance ...
... Classwork – Chemical Quantities (Stoichiometry) 1. Using sandwich making as an analogy to chemical reactions, show the balanced equation that requires 2 pieces of bread, 3 slices of meat and on slice of cheese to make 1 sandwich. 2. Using the ratios for the above process (reaction), show the balance ...
Energetics and Equilibria
... energy, G, and we saw that the Gibbs energy decreases in a spontaneous process. We will have more to say here about the Gibbs energy and how it can be used to understand chemical equilibrium. Ultimately our aim will be to prove the very useful relationship between the standard Gibbs energy change fo ...
... energy, G, and we saw that the Gibbs energy decreases in a spontaneous process. We will have more to say here about the Gibbs energy and how it can be used to understand chemical equilibrium. Ultimately our aim will be to prove the very useful relationship between the standard Gibbs energy change fo ...
Chemical Energy
... The energy released in a chemical reaction raises the internal energy, E, and does work under constant pressure at the expense of energy stored in compounds. Thus, ...
... The energy released in a chemical reaction raises the internal energy, E, and does work under constant pressure at the expense of energy stored in compounds. Thus, ...
Chapter 16 Power Point Notes
... Assessment Questions 2. What causes a gas to expand when its temperature is increased? a. The number of particles increases as temperature increases. b. Each particle expands as its temperature increases, so the total volume increases. c. As temperature increases, more electrons leave atoms and mov ...
... Assessment Questions 2. What causes a gas to expand when its temperature is increased? a. The number of particles increases as temperature increases. b. Each particle expands as its temperature increases, so the total volume increases. c. As temperature increases, more electrons leave atoms and mov ...
Atoms and Elements: Are they Related?
... A burning match is a good example of a chemical reaction. Application of a spark to the chemicals on the match head start the chemical reaction. Signs of a chemical change – heat given off, ...
... A burning match is a good example of a chemical reaction. Application of a spark to the chemicals on the match head start the chemical reaction. Signs of a chemical change – heat given off, ...
Chapter 12: Engineering Thermodynamics
... some way by which both the system and its surroundings can be exactly restored to their respective initial states. A process is irreversible if both the system and surroundings cannot be restored to their initial states. There are many effects whose presence during a process renders it irreversible. ...
... some way by which both the system and its surroundings can be exactly restored to their respective initial states. A process is irreversible if both the system and surroundings cannot be restored to their initial states. There are many effects whose presence during a process renders it irreversible. ...
Chemistry (306) - National Evaluation Series
... bedding, automobile seats, and sponges. An important component in the manufacturing of flexible polyurethane foams are polyols, which are typically derived from petroleum products. In 2007, Cargill, Incorporated, was awarded a Presidential Green Chemistry Challenge Award from the Environmental Prote ...
... bedding, automobile seats, and sponges. An important component in the manufacturing of flexible polyurethane foams are polyols, which are typically derived from petroleum products. In 2007, Cargill, Incorporated, was awarded a Presidential Green Chemistry Challenge Award from the Environmental Prote ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.