LABORATORY MANUAL CHEMISTRY 121
... reaction can be followed visually because the products are red while the reactant is green. The purpose of this exercise is to determine the rate law for the hydrolysis of trans-[Co(en)2Cl2]+ and to determine the activation energy for the hydrolysis reaction by carrying out the reaction at several d ...
... reaction can be followed visually because the products are red while the reactant is green. The purpose of this exercise is to determine the rate law for the hydrolysis of trans-[Co(en)2Cl2]+ and to determine the activation energy for the hydrolysis reaction by carrying out the reaction at several d ...
Page 1 of 7 Chem 1A Exam 2 Review Problems 1. At 0.967 atm, the
... One statement of the first law of thermodynamics is that a. the total energy change for a system is equal to the sum of the heat transferred to or from the system and the work done by or on the system. b. the amount of work done on a system is dependent of pathway. c. the total work done on a ...
... One statement of the first law of thermodynamics is that a. the total energy change for a system is equal to the sum of the heat transferred to or from the system and the work done by or on the system. b. the amount of work done on a system is dependent of pathway. c. the total work done on a ...
Print this article - Bangladesh Journals Online
... assignable for protons Hd and Ha respectively. The two doublets of doublet at δ 6.5 (JHa-Hb = JHb-Hc = J = 8.0 Hz) and 6.9 (JHb-Hc= JHc-Hd = J = 8.0 Hz) accounts for the Ha and Hd respectively, while the relatively downfield signal at δ 8.5 has been assigned for the imine (=N-H) proton of 2-mercapto ...
... assignable for protons Hd and Ha respectively. The two doublets of doublet at δ 6.5 (JHa-Hb = JHb-Hc = J = 8.0 Hz) and 6.9 (JHb-Hc= JHc-Hd = J = 8.0 Hz) accounts for the Ha and Hd respectively, while the relatively downfield signal at δ 8.5 has been assigned for the imine (=N-H) proton of 2-mercapto ...
File
... either is equally acceptable. An example of this is: n textbook — the bombarding electrons in a mass spectrometer have a high kinetic energy n answer — the electrons are fast moving Some answers are expanded to show the logic of the calculation. For instance, the conversion of 23.7 cm3 of solution ...
... either is equally acceptable. An example of this is: n textbook — the bombarding electrons in a mass spectrometer have a high kinetic energy n answer — the electrons are fast moving Some answers are expanded to show the logic of the calculation. For instance, the conversion of 23.7 cm3 of solution ...
Ch 10 - Enrico Fermi High School
... 1. What effect (increase, decrease, no change) will a decrease in temperature have on K? 2. What effect (inc, dec, none) will removing H2 have on the equilibrium constant, K? 3. In which direction will the reaction shift if gaseous H2 is removed from the system? 4. Adding a catalyst (a gold surface) ...
... 1. What effect (increase, decrease, no change) will a decrease in temperature have on K? 2. What effect (inc, dec, none) will removing H2 have on the equilibrium constant, K? 3. In which direction will the reaction shift if gaseous H2 is removed from the system? 4. Adding a catalyst (a gold surface) ...
STOICHIOMETRY REVIEW WORKSHEET
... (d) How much oxygen was used up in moles? (e) How much oxygen was used up in grams? 2) Using the following equation: NaOH + ...
... (d) How much oxygen was used up in moles? (e) How much oxygen was used up in grams? 2) Using the following equation: NaOH + ...
Title Decomposition studies of isopropanol in a
... enough to compete with radical chemistry as a significant process accounting for reactant consumption at the initial stages of reaction. Decomposition and pyrolysis occurs through dehydration and hydrogen elimination reactions, in addition to the usual simple C–C or C-H bond fissions typical of hyd ...
... enough to compete with radical chemistry as a significant process accounting for reactant consumption at the initial stages of reaction. Decomposition and pyrolysis occurs through dehydration and hydrogen elimination reactions, in addition to the usual simple C–C or C-H bond fissions typical of hyd ...
Chemistry - talcher autonomous college
... Kinetic molecular model of a gas: postulates and derivation of the kinetic gas equation; collision frequency; collision diameter; mean free path and viscosity of gases, including their temperature and pressure dependence, relation between mean free path and coefficient of viscosity, calculation of σ ...
... Kinetic molecular model of a gas: postulates and derivation of the kinetic gas equation; collision frequency; collision diameter; mean free path and viscosity of gases, including their temperature and pressure dependence, relation between mean free path and coefficient of viscosity, calculation of σ ...
CfE Advanced Higher Chemistry Unit 2: Organic
... When atoms approach each other, their separate sets of atomic orbitals merge to form a single set of molecular orbitals. Some of the molecular orbitals, known as 'bonding molecular orbitals', occupy the region between two nuclei. The attraction of positive nuclei to negative electrons occupying bond ...
... When atoms approach each other, their separate sets of atomic orbitals merge to form a single set of molecular orbitals. Some of the molecular orbitals, known as 'bonding molecular orbitals', occupy the region between two nuclei. The attraction of positive nuclei to negative electrons occupying bond ...
It`s Easy Being a Green Chemist
... One major goal of Green Chemistry as described by John Warner is to "change the way we make chemists." The traditional process of designing a chemical product typically does not consider the lifecycle of the product or the long-term downstream ramifications of the waste it generates. Too often it is ...
... One major goal of Green Chemistry as described by John Warner is to "change the way we make chemists." The traditional process of designing a chemical product typically does not consider the lifecycle of the product or the long-term downstream ramifications of the waste it generates. Too often it is ...
Principles of Chemistry: A Molecular Approach
... mass and other properties that distinguish them from atoms of other elements. Atoms combine in simple, whole-number ratios to form molecules of compounds. In a chemical reaction, atoms of one element cannot change into atoms of another element. They simply rearrange the way they are attached. ...
... mass and other properties that distinguish them from atoms of other elements. Atoms combine in simple, whole-number ratios to form molecules of compounds. In a chemical reaction, atoms of one element cannot change into atoms of another element. They simply rearrange the way they are attached. ...
mod-5-revision-guide-4-transition-metals
... •Compounds with high oxidation states tend to be oxidising agents e.g MnO4•Compounds with low oxidation states are often reducing agents e.g V2+ & Fe2+ Reducing Chromium Cr3+ (green) and then Cr2+ (blue) are formed by reduction of Cr2O72- (orange) by the strong reducing agent zinc in (HCl) acid solu ...
... •Compounds with high oxidation states tend to be oxidising agents e.g MnO4•Compounds with low oxidation states are often reducing agents e.g V2+ & Fe2+ Reducing Chromium Cr3+ (green) and then Cr2+ (blue) are formed by reduction of Cr2O72- (orange) by the strong reducing agent zinc in (HCl) acid solu ...
Chemistry: An Introduction for Medical and Health Sciences - E
... John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England, or emailed to [email protected], or faxed to (+44) 1243 770620. This publication is designed to provide accurate and authoritative information in regard to the subject matter covered. It is sold on the und ...
... John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England, or emailed to [email protected], or faxed to (+44) 1243 770620. This publication is designed to provide accurate and authoritative information in regard to the subject matter covered. It is sold on the und ...
Chemistry - An Introduction for Medical and Hea..
... John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England, or emailed to [email protected], or faxed to (+44) 1243 770620. This publication is designed to provide accurate and authoritative information in regard to the subject matter covered. It is sold on the und ...
... John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England, or emailed to [email protected], or faxed to (+44) 1243 770620. This publication is designed to provide accurate and authoritative information in regard to the subject matter covered. It is sold on the und ...
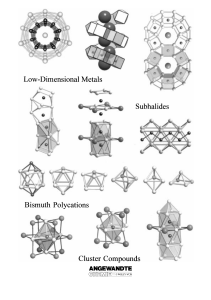
From the Metal to the Molecule
... Mostly, it is found to be more convenient to use intermetallic phases or metal halides as starting materials. In some cases, it is necessary to add auxiliary reagents, such as niobium halides, which modify the partial pressures in the gas phase through various equilibrium processes but are not incor ...
... Mostly, it is found to be more convenient to use intermetallic phases or metal halides as starting materials. In some cases, it is necessary to add auxiliary reagents, such as niobium halides, which modify the partial pressures in the gas phase through various equilibrium processes but are not incor ...
Hadronic Chemistry and Binding Energies
... accepted these notions of so-called well established theory of quantum chemistry. His untiring efforts of a few decades gave birth to the new discipline of Hadronic Chemistry [4]. Hadronic chemistry of small molecules is based on Santilli’s iso- and geno- mathematics by considering the interactions ...
... accepted these notions of so-called well established theory of quantum chemistry. His untiring efforts of a few decades gave birth to the new discipline of Hadronic Chemistry [4]. Hadronic chemistry of small molecules is based on Santilli’s iso- and geno- mathematics by considering the interactions ...
Fluorinated Butatrienes - diss.fu-berlin.de
... stellt sich heraus, dass das Kumulen-Isomer nicht mehr das stabilste Isomer ist. ...
... stellt sich heraus, dass das Kumulen-Isomer nicht mehr das stabilste Isomer ist. ...
Sample pages 2 PDF
... clusters is 2 nm (theoretical calculation gives 1.7 nm). For these particles there is no mode of collective plasmon resonance (Fig. 2.5), i.e. Au25(glutathione)18 clusters show one-electron absorption peaks, while 4-nm nanoparticles are plasmon, and transition from cluster to fcc crystal state takes ...
... clusters is 2 nm (theoretical calculation gives 1.7 nm). For these particles there is no mode of collective plasmon resonance (Fig. 2.5), i.e. Au25(glutathione)18 clusters show one-electron absorption peaks, while 4-nm nanoparticles are plasmon, and transition from cluster to fcc crystal state takes ...
Experiment 7 - MASSIVE REACTIONS
... When elements or molecules combine in chemical reactions, the resulting product(s) remain electrically neutral overall. It’s dynamic and attention-grabbing to start a unit on chemical reactions by illustrating five different types of chemical reactions: synthesis, decomposition, single replacement ( ...
... When elements or molecules combine in chemical reactions, the resulting product(s) remain electrically neutral overall. It’s dynamic and attention-grabbing to start a unit on chemical reactions by illustrating five different types of chemical reactions: synthesis, decomposition, single replacement ( ...
Materials Chemistry Prof. S. Sunder Manoharan Department of
... In general the solid to solid transformations can be studied by Avrami kinetics. So, the nucleation and growth of one solid phase with in the other can be described by this kinetics where random and isolated nucleation at high energy defect sights, with one dimensional two dimensional or three dimen ...
... In general the solid to solid transformations can be studied by Avrami kinetics. So, the nucleation and growth of one solid phase with in the other can be described by this kinetics where random and isolated nucleation at high energy defect sights, with one dimensional two dimensional or three dimen ...
CHM2045 Exam 2 Review Questions Fall 2015
... wavelength 122 mm. Assuming that the specific heat capacity of the soup is the same as that of water (4.184 J/g°C) and no heat loss to the bowl, which choice is closest to the number of photons absorbed? ...
... wavelength 122 mm. Assuming that the specific heat capacity of the soup is the same as that of water (4.184 J/g°C) and no heat loss to the bowl, which choice is closest to the number of photons absorbed? ...
2 - C7Chemistry
... The word stoichiometry comes from the Greek words stoicheion which means “element” and metron which means “measure”. ...
... The word stoichiometry comes from the Greek words stoicheion which means “element” and metron which means “measure”. ...
HONORS LAB MANUAL - Tenafly High School
... 1. Clean a crucible and cover. Dry them by heating in the hottest part of a burner flame for a few minutes. Allow them to cool. Mass and record. 2. Clean a 35-cm length of magnesium ribbon and cut it into small pieces. Place the pieces in the crucible. Mass the crucible, cover and contents. 3. Cover ...
... 1. Clean a crucible and cover. Dry them by heating in the hottest part of a burner flame for a few minutes. Allow them to cool. Mass and record. 2. Clean a 35-cm length of magnesium ribbon and cut it into small pieces. Place the pieces in the crucible. Mass the crucible, cover and contents. 3. Cover ...
Discussion Questions
... amount of titrant has been added to exactly react with the substance being analyzed n Endpoint: the point at which a chemical indicator changes color Oxidation–reduction reactions n Oxidation states are assigned using a set of rules to keep track of electron flow n Oxidation: increase in oxidatio ...
... amount of titrant has been added to exactly react with the substance being analyzed n Endpoint: the point at which a chemical indicator changes color Oxidation–reduction reactions n Oxidation states are assigned using a set of rules to keep track of electron flow n Oxidation: increase in oxidatio ...
Redox

Redox reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between species. The term ""redox"" comes from two concepts involved with electron transfer: reduction and oxidation. It can be explained in simple terms: Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.Although oxidation reactions are commonly associated with the formation of oxides from oxygen molecules, these are only specific examples of a more general concept of reactions involving electron transfer.Redox reactions, or oxidation-reduction reactions, have a number of similarities to acid–base reactions. Like acid–base reactions, redox reactions are a matched set, that is, there cannot be an oxidation reaction without a reduction reaction happening simultaneously. The oxidation alone and the reduction alone are each called a half-reaction, because two half-reactions always occur together to form a whole reaction. When writing half-reactions, the gained or lost electrons are typically included explicitly in order that the half-reaction be balanced with respect to electric charge.Though sufficient for many purposes, these descriptions are not precisely correct. Oxidation and reduction properly refer to a change in oxidation state — the actual transfer of electrons may never occur. The oxidation state of an atom is the fictitious charge that an atom would have if all bonds between atoms of different elements were 100% ionic. Thus, oxidation is better defined as an increase in oxidation state, and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always cause a change in oxidation state, but there are many reactions that are classed as ""redox"" even though no electron transfer occurs (such as those involving covalent bonds).There are simple redox processes, such as the oxidation of carbon to yield carbon dioxide (CO2) or the reduction of carbon by hydrogen to yield methane (CH4), and more complex processes such as the oxidation of glucose (C6H12O6) in the human body through a series of complex electron transfer processes.