Review Final 111 Lect
... examples of an element or a compound. 17. Which of the 0.010 m solution given below : K3PO4 , C2H6O(alcohol) , HCN , NaOH, (NH4)2SO4 would have: a. The highest boiling point __________ b. The lowest freezing point. _________ Explain your answer. ______________________________________________________ ...
... examples of an element or a compound. 17. Which of the 0.010 m solution given below : K3PO4 , C2H6O(alcohol) , HCN , NaOH, (NH4)2SO4 would have: a. The highest boiling point __________ b. The lowest freezing point. _________ Explain your answer. ______________________________________________________ ...
Question 1. Phosgene was used during the World War - IQ
... (b) Azobenzene is known to have greater delocalization of its π electrons than the hydrazobenzene. Provide an explanation for this statement based on non-hybridized atomic orbitals and N-N-C bond angles in each of these molecules. Question 9. The molecule of ethyl butanoate (C6H12O2) is responsible ...
... (b) Azobenzene is known to have greater delocalization of its π electrons than the hydrazobenzene. Provide an explanation for this statement based on non-hybridized atomic orbitals and N-N-C bond angles in each of these molecules. Question 9. The molecule of ethyl butanoate (C6H12O2) is responsible ...
Teacher Guide - Price9thScience
... a projector and do the Exploration as a teacher-led activity. (Note: Calculators would be very helpful for calculating density. If students do not have their own, they can use the calculators on their computers.) 4. Discussion questions ( 15 – 30 minutes) As students are working or just after they a ...
... a projector and do the Exploration as a teacher-led activity. (Note: Calculators would be very helpful for calculating density. If students do not have their own, they can use the calculators on their computers.) 4. Discussion questions ( 15 – 30 minutes) As students are working or just after they a ...
Pdf
... adjusted ee, | ee adj | . This amounts over an ensemble of many repetitions to a persistent vividly bimodal ee distribution for C monomers, that is, the effectiveness of a "monomer purification" mechanism. Of course the number of unbound product molecules is declining slowly over time as the system ...
... adjusted ee, | ee adj | . This amounts over an ensemble of many repetitions to a persistent vividly bimodal ee distribution for C monomers, that is, the effectiveness of a "monomer purification" mechanism. Of course the number of unbound product molecules is declining slowly over time as the system ...
Advanced Placement Chemistry: 1984 Free Response Questions
... (b) Burning coal containing a significant amount of sulfur leads to "acid rain." (c) Perspiring is a mechanism for cooling the body. (d) The addition of antifreeze to water in a radiator decreases the likelihood that the liquid in the radiator will either freeze or boil. ...
... (b) Burning coal containing a significant amount of sulfur leads to "acid rain." (c) Perspiring is a mechanism for cooling the body. (d) The addition of antifreeze to water in a radiator decreases the likelihood that the liquid in the radiator will either freeze or boil. ...
Multiple Choice Practice. A) P B) S C) Cl D) Li E) 1 F 1. Has the
... When the half reaction above is balanced, how many moles of electrons are needed for every mole of I2 formed by this half-reaction? A) 2 B) 6 C) 8 D) 10 E) 12 30. Which of the following is always true at the triple point of a pure substance? A) The vapor pressure of the solid phase equals the vapor ...
... When the half reaction above is balanced, how many moles of electrons are needed for every mole of I2 formed by this half-reaction? A) 2 B) 6 C) 8 D) 10 E) 12 30. Which of the following is always true at the triple point of a pure substance? A) The vapor pressure of the solid phase equals the vapor ...
Chapter 13 Rocks and Minerals
... Mineral Formation Minerals form in many different ways. - melted rock inside the earth (magma). - melted rock that reaches the earths surface (lava). -evaporation of water leaving minerals behind. (ocean water, salt in cup after ocean water evaporates) - precipitation of minerals suspended in water ...
... Mineral Formation Minerals form in many different ways. - melted rock inside the earth (magma). - melted rock that reaches the earths surface (lava). -evaporation of water leaving minerals behind. (ocean water, salt in cup after ocean water evaporates) - precipitation of minerals suspended in water ...
Numerical Analysis of Fifth-Harmonic Conversion of Low
... crystal A, which generates the second harmonic. The second harmonic is sent through crystal B where the fourth harmonic is generated. The remaining second harmonic is directed back into crystal A after a time delay equal to the roundtrip time of the ring cavity. The leftover fundamental is allowed t ...
... crystal A, which generates the second harmonic. The second harmonic is sent through crystal B where the fourth harmonic is generated. The remaining second harmonic is directed back into crystal A after a time delay equal to the roundtrip time of the ring cavity. The leftover fundamental is allowed t ...
Double-etch geometry for millimeter-wave photonic band
... If the measurement does not result in. the desired band-gap frequencies (if the measured value is less than the desired value), the wafers can be put back into the etch solution for more overetching. After the overetch, they can be restacked and measured again. This process can be repeated until the ...
... If the measurement does not result in. the desired band-gap frequencies (if the measured value is less than the desired value), the wafers can be put back into the etch solution for more overetching. After the overetch, they can be restacked and measured again. This process can be repeated until the ...
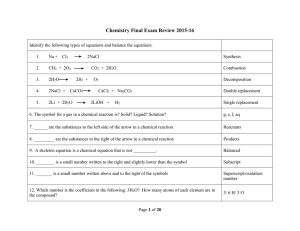
Type Of Chemical Reaction
... A strong base is typically a pH of what number? _____14_____ The ion that accounts for Acidity is: _____H+_or H3O+____ The ion that accounts for Basic is: ____OH-____ ...
... A strong base is typically a pH of what number? _____14_____ The ion that accounts for Acidity is: _____H+_or H3O+____ The ion that accounts for Basic is: ____OH-____ ...
Diphenyldichlorophosphonium Trichloride−Chlorine Solvate 1:1
... that the stability of the ionic form of diphenyltrichlorophosphorane is closely related to excess chlorine, which was used during the synthesis. Our findings are interesting also from another point of view: while triiodide and tribromide anions are well-known and can easily be obtained by addition o ...
... that the stability of the ionic form of diphenyltrichlorophosphorane is closely related to excess chlorine, which was used during the synthesis. Our findings are interesting also from another point of view: while triiodide and tribromide anions are well-known and can easily be obtained by addition o ...
Igneous Rocks
... are called extrusive. Where they form is crucial in their identification. Those that form within the earth cool slowly due to the earth’s heat. During that interval, which can last years, minerals in the magma migrate and are able to join with similar molecules to form crystals. The more time that t ...
... are called extrusive. Where they form is crucial in their identification. Those that form within the earth cool slowly due to the earth’s heat. During that interval, which can last years, minerals in the magma migrate and are able to join with similar molecules to form crystals. The more time that t ...
Magnesite MgCO3
... Stevens Co., Washington. Immense deposits in the Liaoning district, Liaoning Province, China. Numerous other occurrences are known. Name: ...
... Stevens Co., Washington. Immense deposits in the Liaoning district, Liaoning Province, China. Numerous other occurrences are known. Name: ...
1.2 The mass and size of the atom
... Schematic of a quadrupole mass filter. The ion beam, moving in the +z direction, is deflected by a high-frequency alternating potential. In order for the beam to pass through the filter, a certain relation between e/m, the frequency , and the deflection voltages U and V must be fulfilled. The dashe ...
... Schematic of a quadrupole mass filter. The ion beam, moving in the +z direction, is deflected by a high-frequency alternating potential. In order for the beam to pass through the filter, a certain relation between e/m, the frequency , and the deflection voltages U and V must be fulfilled. The dashe ...
Parametric generation of tunable infrared radiation in
... type-I phase matching the OPG linewidth is larger than in type II, especially near degeneracy. The walk-off length is given by pL, where p is a Poyntingvector walk-off angle'3 p (An/n)sin(20); An is a birefringence value and n is the mean refractive index. The effective crystal length is calculated ...
... type-I phase matching the OPG linewidth is larger than in type II, especially near degeneracy. The walk-off length is given by pL, where p is a Poyntingvector walk-off angle'3 p (An/n)sin(20); An is a birefringence value and n is the mean refractive index. The effective crystal length is calculated ...
Minerals - Madison Public Schools
... • A mineral is a naturally formed, inorganic solid that has a definite crystalline structure. • All minerals contain one or more of the 92 naturally occurring elements. ...
... • A mineral is a naturally formed, inorganic solid that has a definite crystalline structure. • All minerals contain one or more of the 92 naturally occurring elements. ...
Chemistry 1: Second Semester Practice Exam Read each question
... B. 22.4 liters at 2 atmospheres D. 11.2 liters at 2 atmospheres 5. At STP, 1 liter of oxygen gas would have the same number of molecules as A. 1 Liter of H2 C. 3 Liters of CO2 B. 2 Liters of CO D. 3 Liters of Ne 6. As the temperature of a gas increases with the volume remaining constant the pressure ...
... B. 22.4 liters at 2 atmospheres D. 11.2 liters at 2 atmospheres 5. At STP, 1 liter of oxygen gas would have the same number of molecules as A. 1 Liter of H2 C. 3 Liters of CO2 B. 2 Liters of CO D. 3 Liters of Ne 6. As the temperature of a gas increases with the volume remaining constant the pressure ...
Mineral & Rock
... solid definite chemical composition crystal structure. **It must have all five of the characteristics described in this definition.** ...
... solid definite chemical composition crystal structure. **It must have all five of the characteristics described in this definition.** ...
Lab 2: Igneous/Metamorphic Rocks
... Formed through process of metamorphism Transition of one rock into another by increasing ...
... Formed through process of metamorphism Transition of one rock into another by increasing ...
Valence and crystal structure - IDC
... Neutral Sodium atom donates an electron to neutral Chlorine atom forming Na+ and Cl- ions. Sodium chloride crystallizes in the cubic structure shown in Figure below. This model is not to scale to show the three dimensional structure. The Na+Cl- ions are actually packed similar to layers of stacked m ...
... Neutral Sodium atom donates an electron to neutral Chlorine atom forming Na+ and Cl- ions. Sodium chloride crystallizes in the cubic structure shown in Figure below. This model is not to scale to show the three dimensional structure. The Na+Cl- ions are actually packed similar to layers of stacked m ...
Chapter 3 Vocabulary List
... Basaltic composition- A compositional group of igneous rocks indicating that the rock contains substantial dark silicate minerals and calcium-rich plagioclase feldspar Bowen’s reaction series- A concept proposed by N.L. Bowen that illustrates the relationships between magma and the minerals crystall ...
... Basaltic composition- A compositional group of igneous rocks indicating that the rock contains substantial dark silicate minerals and calcium-rich plagioclase feldspar Bowen’s reaction series- A concept proposed by N.L. Bowen that illustrates the relationships between magma and the minerals crystall ...
AL COS #
... What term describes two liquids that can be mixed together but Immiscible separate shortly after you cease mixing them? What term describes two liquids that are soluble in each other? Miscible What is the substance that is dissolved in a solution? Solute What is the substance that dissolves a solute ...
... What term describes two liquids that can be mixed together but Immiscible separate shortly after you cease mixing them? What term describes two liquids that are soluble in each other? Miscible What is the substance that is dissolved in a solution? Solute What is the substance that dissolves a solute ...
CHEMISTRY: Practice Spring Final
... 28) What happens to the water in a calorimeter when an exothermic reaction occurs in it? A) It absorbs heat, and a drop in temperature is observed. B) It absorbs heat, and a rise in temperature is observed. C) It releases heat, and a drop in temperature is observed. D) It releases heat, and a rise i ...
... 28) What happens to the water in a calorimeter when an exothermic reaction occurs in it? A) It absorbs heat, and a drop in temperature is observed. B) It absorbs heat, and a rise in temperature is observed. C) It releases heat, and a drop in temperature is observed. D) It releases heat, and a rise i ...
File
... C) 8 protons and 5 neutrons D) 7 protons and 7 neutrons E) 6 protons and 8 neutrons 40. Which of the following statements about atoms is incorrect? A) Atoms are neutral because they contain the same number of protons and electrons B) All atoms of a given element must contain the same number protons, ...
... C) 8 protons and 5 neutrons D) 7 protons and 7 neutrons E) 6 protons and 8 neutrons 40. Which of the following statements about atoms is incorrect? A) Atoms are neutral because they contain the same number of protons and electrons B) All atoms of a given element must contain the same number protons, ...
Crystallization

Crystallization is the (natural or artificial) process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore an aspect of precipitation, obtained through a variation of the solubility conditions of the solute in the solvent, as compared to precipitation due to chemical reaction.