M for Moles - Shop
... A chemical equation is an important piece of information for most of the moles calculations. A chemical equation shows a complete summary description of a chemical reaction. However, before the equation can be used, it must be properly balanced. Balancing a chemical equation involves two parts: (1) ...
... A chemical equation is an important piece of information for most of the moles calculations. A chemical equation shows a complete summary description of a chemical reaction. However, before the equation can be used, it must be properly balanced. Balancing a chemical equation involves two parts: (1) ...
Balancing Chemical Equations Academic Success Center Science Tutoring Area *
... 1.Write the correct formula for each of the reactants and products 2.Verify the net ionic charge of each of the reactants and products is balanced. If it is not balance it using subscripts ...
... 1.Write the correct formula for each of the reactants and products 2.Verify the net ionic charge of each of the reactants and products is balanced. If it is not balance it using subscripts ...
Chapter 7
... • Once you know the molar mass of a substance, you can convert moles of that substance into mass (or mass into moles). • Carbon has a molar mass of 12.0 grams. • How many moles are in 22 grams of carbon? • 22 g X 1 mol/12.0 g = 1.83 moles or 1.8 moles in significant digits • mol is the abbreviation ...
... • Once you know the molar mass of a substance, you can convert moles of that substance into mass (or mass into moles). • Carbon has a molar mass of 12.0 grams. • How many moles are in 22 grams of carbon? • 22 g X 1 mol/12.0 g = 1.83 moles or 1.8 moles in significant digits • mol is the abbreviation ...
3.091 – Introduction to Solid State Chemistry Lecture Notes No
... Since atoms of each of the elements have different electronic structures, the variety of possible chemical bonds (differing from each other in at least some small way) is considerable and is even further increased by the effects of neighboring atoms on the bond under consideration. The modes of bond ...
... Since atoms of each of the elements have different electronic structures, the variety of possible chemical bonds (differing from each other in at least some small way) is considerable and is even further increased by the effects of neighboring atoms on the bond under consideration. The modes of bond ...
Unit 4, Lesson #3 - Patterson Science
... The value of Keq is determined experimentally. Chemists allow reactions to occur at stated temperatures, until the system no longer changes. At this point, they measure the amounts of both the reactants and products. Just as chemists monitor changes in pH, colour, gas pressure or conductivity of sol ...
... The value of Keq is determined experimentally. Chemists allow reactions to occur at stated temperatures, until the system no longer changes. At this point, they measure the amounts of both the reactants and products. Just as chemists monitor changes in pH, colour, gas pressure or conductivity of sol ...
Chapter Six
... kJ/mol, must be metabolized by a 55-kg person with hypothermia (low body temperature) to raise the temperature of the body water from 33.5 °C to the normal body temperature of 37.0 °C? Assume the products of metabolism are in their most stable states at 25 °C. What volume of air at 37 °C having a pa ...
... kJ/mol, must be metabolized by a 55-kg person with hypothermia (low body temperature) to raise the temperature of the body water from 33.5 °C to the normal body temperature of 37.0 °C? Assume the products of metabolism are in their most stable states at 25 °C. What volume of air at 37 °C having a pa ...
ANALYSIS OF THE SILVER GROUP CATIONS
... may be possible to test for one particular ion in the presence of just one or two others. Alternatively, each subgroup of just a few ions may be separated further so that each ion in the subgroup ends up in a different test tube where its presence can be confirmed by other chemical tests. The chemic ...
... may be possible to test for one particular ion in the presence of just one or two others. Alternatively, each subgroup of just a few ions may be separated further so that each ion in the subgroup ends up in a different test tube where its presence can be confirmed by other chemical tests. The chemic ...
Chapter Six - DePaul University Department of Chemistry
... The internal energy of a fixed quantity of an ideal gas depends only on its temperature. If a sample of an ideal gas is allowed to expand against a constant pressure at a constant temperature, (a) what is DU for the gas? (b) Does the gas do work? (c) Is any heat exchanged with the surroundings? ...
... The internal energy of a fixed quantity of an ideal gas depends only on its temperature. If a sample of an ideal gas is allowed to expand against a constant pressure at a constant temperature, (a) what is DU for the gas? (b) Does the gas do work? (c) Is any heat exchanged with the surroundings? ...
Chemistry 12 is an intensive course, covering a great deal of
... Assignments will be given daily. Students are responsible for catching up and completing any missed assignments due to absences as soon as possible. Missed tests must be made up the next class present (within a one week period) or be given a mark of zero or omitted at the discretion of the teacher. ...
... Assignments will be given daily. Students are responsible for catching up and completing any missed assignments due to absences as soon as possible. Missed tests must be made up the next class present (within a one week period) or be given a mark of zero or omitted at the discretion of the teacher. ...
Maths for Chemistry Facts and Formulae
... we obtain the ideal gas law. Van der Waals equation takes into account the finite distance between molecules and interparticle attractions: ...
... we obtain the ideal gas law. Van der Waals equation takes into account the finite distance between molecules and interparticle attractions: ...
Chemistry Lab 2010
... to change initial concentrations of each reactant. • Measure how initial rate changes as concentration of each reactant is changed. • Zeroth order = concentration doubles, rate unchanged • First order = concentration doubles, rate doubles • Second order = concentration doubles, rate quadruples Remem ...
... to change initial concentrations of each reactant. • Measure how initial rate changes as concentration of each reactant is changed. • Zeroth order = concentration doubles, rate unchanged • First order = concentration doubles, rate doubles • Second order = concentration doubles, rate quadruples Remem ...
4 Expressing and Measuring Chemical Change
... new properties and their own unique chemical formulas. Such changes involve the breaking and forming of chemical bonds. They are referred to as chemical reactions. The processes of photosynthesis and aerobic cellular respiration, for example, involve a series of chemical reactions that produce and u ...
... new properties and their own unique chemical formulas. Such changes involve the breaking and forming of chemical bonds. They are referred to as chemical reactions. The processes of photosynthesis and aerobic cellular respiration, for example, involve a series of chemical reactions that produce and u ...
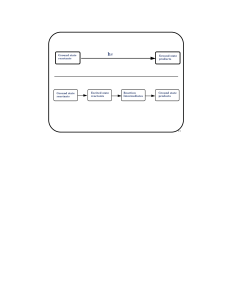
Ground state reactants Ground state products Ground state
... Energy levels for molecular oxygen. Excited triplet states have not been included because they are much higher in energy. The 1∆g state is the one normally refereed to as singlet oxygen. ...
... Energy levels for molecular oxygen. Excited triplet states have not been included because they are much higher in energy. The 1∆g state is the one normally refereed to as singlet oxygen. ...
all practice examples
... A piece of titanium metal with a mass of 20.8 g is heated in boiling water to 99.5 °C and then dropped into a coffee-cup calorimeter containing 75.0 g of water at 21.7 °C. When thermal equilibrium is reached, the final temperature is 24.3 °C. Calculate the specific heat capacity of titanium. (Specif ...
... A piece of titanium metal with a mass of 20.8 g is heated in boiling water to 99.5 °C and then dropped into a coffee-cup calorimeter containing 75.0 g of water at 21.7 °C. When thermal equilibrium is reached, the final temperature is 24.3 °C. Calculate the specific heat capacity of titanium. (Specif ...
Direct Energy Conversion: Fuel Cells
... Solid oxide fuel cells (SOFC) can also utilize carbon monoxide (CO). This makes them more fuel flexible and also generally more efficient with available fuels, such as natural gas or propane. Hydrogen and CO can be produced from natural gas and other fuels by steam reforming, for example. Fuel cells ...
... Solid oxide fuel cells (SOFC) can also utilize carbon monoxide (CO). This makes them more fuel flexible and also generally more efficient with available fuels, such as natural gas or propane. Hydrogen and CO can be produced from natural gas and other fuels by steam reforming, for example. Fuel cells ...
Reactions of Plutonium Dioxide with Water and Oxygen
... (approximately 0.1 g) were accurately weighed prior to placement in the Pt sample container. The oxide was outgassed to constant mass in vacuum at 400eC and was then exposed to water pressure at 15 tom as the temperature was increased stepwise from 25°C to 50, 100, 150, and 250°C over a period in ex ...
... (approximately 0.1 g) were accurately weighed prior to placement in the Pt sample container. The oxide was outgassed to constant mass in vacuum at 400eC and was then exposed to water pressure at 15 tom as the temperature was increased stepwise from 25°C to 50, 100, 150, and 250°C over a period in ex ...
Lab 6
... Solubility in which simple solubility or miscibility due to Dipole-dipole Interaction, Hydrogen bonding - special case of dipole-dipole when there is a hydrogen bonded to a N, O, or F. (or Ion-dipole - interaction of an ion with a polar molecule) examples: dissolving any ionic compound in water), su ...
... Solubility in which simple solubility or miscibility due to Dipole-dipole Interaction, Hydrogen bonding - special case of dipole-dipole when there is a hydrogen bonded to a N, O, or F. (or Ion-dipole - interaction of an ion with a polar molecule) examples: dissolving any ionic compound in water), su ...
practice unit #2 exam
... T2 is the new curve – the entire graph shifts to the right as all particles have more energy at the higher temperature. ...
... T2 is the new curve – the entire graph shifts to the right as all particles have more energy at the higher temperature. ...
Chemistry Worksheets
... Compound B is burned in a bomb calorimeter that contains 1.50 liters of water. When I burned 50.0 grams of compound B in the calorimeter, the temperature rise of the water in the calorimeter was 35.00 C. If the heat of combustion of compound B is 2,150 kJ/mol, what is the molar mass of compound B? ...
... Compound B is burned in a bomb calorimeter that contains 1.50 liters of water. When I burned 50.0 grams of compound B in the calorimeter, the temperature rise of the water in the calorimeter was 35.00 C. If the heat of combustion of compound B is 2,150 kJ/mol, what is the molar mass of compound B? ...
as a PDF
... which form two-electron metals. In these two cases, reaction 6 is one in which the number of 4f electrons decreases by one. We can assume that the energy variation would be smooth if europium and ytterbium were three-electron metals like the other lanthanides. The observed deviations of about 85 and ...
... which form two-electron metals. In these two cases, reaction 6 is one in which the number of 4f electrons decreases by one. We can assume that the energy variation would be smooth if europium and ytterbium were three-electron metals like the other lanthanides. The observed deviations of about 85 and ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.