Chemistry - Beachwood City Schools
... are mixed. If a precipitate forms, write a net ionic equation for the reaction. a) iron(III) nitrate and potassium hydroxide b) ammonium chloride and lithium carbonate c) sodium sulfide and nickel(II) sulfate 3. For each of the following equations i. indicate whether it is a combustion (C), synthesi ...
... are mixed. If a precipitate forms, write a net ionic equation for the reaction. a) iron(III) nitrate and potassium hydroxide b) ammonium chloride and lithium carbonate c) sodium sulfide and nickel(II) sulfate 3. For each of the following equations i. indicate whether it is a combustion (C), synthesi ...
ChemFinalgeocities
... One isotope of carbon has 6 protons and 6 neutrons. The number of protons and neutrons of a second isotope of carbon would be _____. a. 7 and 6 c. 7 and 7 b. 6 and 7 d. 6 and 6 According to the law of conservation of matter, if 4.0 g of hydrogen react with chlorine to produce 146 g of hydrogen chlor ...
... One isotope of carbon has 6 protons and 6 neutrons. The number of protons and neutrons of a second isotope of carbon would be _____. a. 7 and 6 c. 7 and 7 b. 6 and 7 d. 6 and 6 According to the law of conservation of matter, if 4.0 g of hydrogen react with chlorine to produce 146 g of hydrogen chlor ...
0 Quarter Three Assessment Review - SRHSchem
... the solid is completely dissolve, the water temperature drops to 15.2°C. a. Is this process endothermic or exothermic? Explain. – The temperature of the water decreases, so the dissolving process must have absorbed that heat, ...
... the solid is completely dissolve, the water temperature drops to 15.2°C. a. Is this process endothermic or exothermic? Explain. – The temperature of the water decreases, so the dissolving process must have absorbed that heat, ...
CHEMISTRY
... Metals – greater activity = greater ease to lose e Non-metals – greater activity = greater ease to gain e Order is determined by single-replacement ...
... Metals – greater activity = greater ease to lose e Non-metals – greater activity = greater ease to gain e Order is determined by single-replacement ...
Salt Marshes II
... solute concentration to a region of high solute concentration, or in other words, from a high water concentration to a low water concentration. The selectively-permeable membrane is permeable to the solvent, but not to the solute, resulting in a chemical potential difference across the membrane whic ...
... solute concentration to a region of high solute concentration, or in other words, from a high water concentration to a low water concentration. The selectively-permeable membrane is permeable to the solvent, but not to the solute, resulting in a chemical potential difference across the membrane whic ...
worksheet Ka Kb buffers Ksp
... b. If a reaction occurs, how fast will it occur? c. What is the mechanism by which the reaction occurs? d. If substances react, what energy changes are associated with the reaction? ...
... b. If a reaction occurs, how fast will it occur? c. What is the mechanism by which the reaction occurs? d. If substances react, what energy changes are associated with the reaction? ...
Types of Reactions Lab
... HCl: Corrosive! Harmful if ingested (burning of mouth, throat, etc.). Swallowing may be fatal. Skin & eye contact may cause severe burns depending on concentration. Wash skin/eyes for 15 minutes if contact occurs. Ethanol: Flammable! Low flash point and high evaporation rates require extreme caution ...
... HCl: Corrosive! Harmful if ingested (burning of mouth, throat, etc.). Swallowing may be fatal. Skin & eye contact may cause severe burns depending on concentration. Wash skin/eyes for 15 minutes if contact occurs. Ethanol: Flammable! Low flash point and high evaporation rates require extreme caution ...
Thermochemistry (Energy Relationships in Chemical Reactions
... high to be comfortable by virtue of their temperature. But the room temperature glass of water formed from either initial state would be exactly the same, and just as drinkable. The State of the water does not depend on where or what its been, just what it is now. Any property that does not depend o ...
... high to be comfortable by virtue of their temperature. But the room temperature glass of water formed from either initial state would be exactly the same, and just as drinkable. The State of the water does not depend on where or what its been, just what it is now. Any property that does not depend o ...
2014 Academic Challenge Sectional Chemistry Exam Solution Set 1
... D. The balanced reaction is: 2CuO(s) + C(s) 2Cu(s) + CO2(g). Using molecular weights to convert the given mass to moles, there are 1.26 moles of CuO. Using the stoichiometry of the reaction: 1 mol CO ...
... D. The balanced reaction is: 2CuO(s) + C(s) 2Cu(s) + CO2(g). Using molecular weights to convert the given mass to moles, there are 1.26 moles of CuO. Using the stoichiometry of the reaction: 1 mol CO ...
Chemical Reactions and Stoichiometry
... VII. Types of Chemical Reactions - Doing reactions in a lab can be dangerous, time consuming and/or expensive. Recognizing patterns allows scientists to predict the products of many reactions. a. Synthesis – also called combination reactions. Two or more reactants form a single product. Reactants ar ...
... VII. Types of Chemical Reactions - Doing reactions in a lab can be dangerous, time consuming and/or expensive. Recognizing patterns allows scientists to predict the products of many reactions. a. Synthesis – also called combination reactions. Two or more reactants form a single product. Reactants ar ...
chemistry - ALLEN Jaipur
... In an ionic compound the anion (N −) from cubic close type of packing. While the cation (M+) ions occupy one third of the tetrahedral voids. Deduce the empirical formula of the compound and the coordination number of (M+) ions. ...
... In an ionic compound the anion (N −) from cubic close type of packing. While the cation (M+) ions occupy one third of the tetrahedral voids. Deduce the empirical formula of the compound and the coordination number of (M+) ions. ...
Document
... Hydrogen ions, H+(aq), make solutions acidic and hydroxide ions, OH–(aq), make solutions alkaline. The pH scale is a measure of the acidity or alkalinity of a solution. In neutralisation reactions, hydrogen ions react with hydroxide ions to produce water. This reaction can be represented by the equa ...
... Hydrogen ions, H+(aq), make solutions acidic and hydroxide ions, OH–(aq), make solutions alkaline. The pH scale is a measure of the acidity or alkalinity of a solution. In neutralisation reactions, hydrogen ions react with hydroxide ions to produce water. This reaction can be represented by the equa ...
- Deans Community High School
... 1. Controlling the Rate of Reaction 1. Two identical samples of zinc were added to an excess of two solutions of sulphuric acid, concentrations 2 mol l-1 and 1 mol l-1 respectively. Which of the following would have been the same for the two samples? A. The total mass lost B. The total time for the ...
... 1. Controlling the Rate of Reaction 1. Two identical samples of zinc were added to an excess of two solutions of sulphuric acid, concentrations 2 mol l-1 and 1 mol l-1 respectively. Which of the following would have been the same for the two samples? A. The total mass lost B. The total time for the ...
Chemistry Exam 2 Specifications and Sample Exam
... iii. Give one reason why this might be important to the continued operation of the cell. ...
... iii. Give one reason why this might be important to the continued operation of the cell. ...
LIQUIDS
... After element 20 the electron arrangement becomes more complicated, but it is always true that elements in Group 1 have one electron in their outer shell, so we can say that Rb, Cs and Fr will all have one electron in their outer shell. Therefore elements in Group 3 always have three electrons in th ...
... After element 20 the electron arrangement becomes more complicated, but it is always true that elements in Group 1 have one electron in their outer shell, so we can say that Rb, Cs and Fr will all have one electron in their outer shell. Therefore elements in Group 3 always have three electrons in th ...
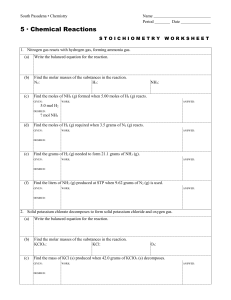
Chapter 12 Review “Stoichiometry”
... g water, 2.24 L of hydrogen gas forms (at STP). How would the amount of hydrogen produced change if the volume of water was decreased to 440 mL (440 g)? When two substances react to form products, the reactant which is used up is called the ____. ...
... g water, 2.24 L of hydrogen gas forms (at STP). How would the amount of hydrogen produced change if the volume of water was decreased to 440 mL (440 g)? When two substances react to form products, the reactant which is used up is called the ____. ...
Chapter 12 Review “Stoichiometry”
... g water, 2.24 L of hydrogen gas forms (at STP). How would the amount of hydrogen produced change if the volume of water was decreased to 440 mL (440 g)? When two substances react to form products, the reactant which is used up is called the ____. ...
... g water, 2.24 L of hydrogen gas forms (at STP). How would the amount of hydrogen produced change if the volume of water was decreased to 440 mL (440 g)? When two substances react to form products, the reactant which is used up is called the ____. ...
AP Chem
... .2M MgCl2. What is the final concentration of Pb2+ ions in the solution? A. .2M B. .1M C. .05M D. .025M E. .012M 20. One half liter of .2M HCl is added to one half liter of .4M AgNO3. What is the mass of AgCl produced? A. 14g B. 28g C. 42g D. 70g E. 84g 21. The first ionization energy for Mg is 730k ...
... .2M MgCl2. What is the final concentration of Pb2+ ions in the solution? A. .2M B. .1M C. .05M D. .025M E. .012M 20. One half liter of .2M HCl is added to one half liter of .4M AgNO3. What is the mass of AgCl produced? A. 14g B. 28g C. 42g D. 70g E. 84g 21. The first ionization energy for Mg is 730k ...
PowerPoint for Cornell Notes
... • Neutralization is a type of chemical reaction in which a strong acid and strong base react with each other to form water and salt. Have you ever been unlucky enough to be stung by a wasp or a bee? Bee stings are acidic in nature, which is why a household remedy for a bee sting is baking soda or so ...
... • Neutralization is a type of chemical reaction in which a strong acid and strong base react with each other to form water and salt. Have you ever been unlucky enough to be stung by a wasp or a bee? Bee stings are acidic in nature, which is why a household remedy for a bee sting is baking soda or so ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.