Fe(H2O)63+ + H2O → ← H3O+ + Fe(H2O)5(OH)2+
... For a substance to be considered a strong electrolyte, it must (A) (B) (C) (D) ...
... For a substance to be considered a strong electrolyte, it must (A) (B) (C) (D) ...
Solid-state electrochemical gas sensors P. J
... solid electrolytes [19–24] and is based on the growth and decomposition of the gassensitive layer and the reactivity of this layer with the surrounding gas. Figure 6 presents the structure of an electrocatalytic sensor based on Lisicon solid electrolyte [25]. When a voltage ramp is applied to the se ...
... solid electrolytes [19–24] and is based on the growth and decomposition of the gassensitive layer and the reactivity of this layer with the surrounding gas. Figure 6 presents the structure of an electrocatalytic sensor based on Lisicon solid electrolyte [25]. When a voltage ramp is applied to the se ...
Energy transfer
... Example: ice melting H2O(s) H20(l) There is no temperature change. Energy is used to overcome intermolecular attractions. ...
... Example: ice melting H2O(s) H20(l) There is no temperature change. Energy is used to overcome intermolecular attractions. ...
Complete ionic equation
... Diatomic Molecules • Remember which atoms make diatomic molecules: – H2 and N2, O2, F2 Cl2 Br2 I2 -This is only when they are by themselves! -When other atoms are by themselves they don’t have any subscripts, for example iron is just Fe ...
... Diatomic Molecules • Remember which atoms make diatomic molecules: – H2 and N2, O2, F2 Cl2 Br2 I2 -This is only when they are by themselves! -When other atoms are by themselves they don’t have any subscripts, for example iron is just Fe ...
PPT: Chemical Reactions Review
... (CO3)2-: What is the oxidation # of C? O is -2, and the overall charge is -2 So C + 3(O) = -2 or C + 3(-2) = -2 C = +4 The oxidation # of ions = charge of ions Mn3+ has an oxidation # of +3 S2- has an oxidation # of -2 ...
... (CO3)2-: What is the oxidation # of C? O is -2, and the overall charge is -2 So C + 3(O) = -2 or C + 3(-2) = -2 C = +4 The oxidation # of ions = charge of ions Mn3+ has an oxidation # of +3 S2- has an oxidation # of -2 ...
Many thermal and chemical reactions occur during the roasting
... F, carmelization begins. It is at this point that water and carbon dioxide fracture and outgassing begins causing the first mechanical crack. These are the chemical reactions, occurring at approximately 356 degrees F, that are exothermic. Once carmelization begins, it is very important that the coff ...
... F, carmelization begins. It is at this point that water and carbon dioxide fracture and outgassing begins causing the first mechanical crack. These are the chemical reactions, occurring at approximately 356 degrees F, that are exothermic. Once carmelization begins, it is very important that the coff ...
Chapter 7-8-9
... temperature of potassium chloride? a. The melting temperature is relatively high. b. The melting temperature is variable and unpredictable. c. The melting temperature is relatively low. d. Potassium chloride does not melt. Which of the following pairs of elements is most likely to form a covalent co ...
... temperature of potassium chloride? a. The melting temperature is relatively high. b. The melting temperature is variable and unpredictable. c. The melting temperature is relatively low. d. Potassium chloride does not melt. Which of the following pairs of elements is most likely to form a covalent co ...
Unit 3 Homework Booklet
... How many grams of magnesium oxide would be produced by reacting completely 4.0 g of magnesium with oxygen? ...
... How many grams of magnesium oxide would be produced by reacting completely 4.0 g of magnesium with oxygen? ...
TiO2-Organics
... inconveniences and detrimental factors in direct solar photolysis are the lack of sunlight absorption by the substrates, attenuation of the sunlight, and the relatively shallow penetration depth of sunlight in natural aquatic bodies. There are many types of catalyst, some act on very few substrates ...
... inconveniences and detrimental factors in direct solar photolysis are the lack of sunlight absorption by the substrates, attenuation of the sunlight, and the relatively shallow penetration depth of sunlight in natural aquatic bodies. There are many types of catalyst, some act on very few substrates ...
PHYSICAL SETTING CHEMISTRY
... KAl(SO4)2 •12H2O. It is a hydrated compound because water molecules are included within its crystal structure. There are 12 moles of H2O for every 1 mole of KAl(SO4)2. The compound contains two different positive ions. The gram-formula mass of KAl(SO4)2 •12H2O is 474 grams per mole. 66 Identify one ...
... KAl(SO4)2 •12H2O. It is a hydrated compound because water molecules are included within its crystal structure. There are 12 moles of H2O for every 1 mole of KAl(SO4)2. The compound contains two different positive ions. The gram-formula mass of KAl(SO4)2 •12H2O is 474 grams per mole. 66 Identify one ...
Exam 3 - Canvas by Instructure
... D. Cannot determine from information provided. 6. Once nitrogen dioxide is generated, it combines with water to generate nitric acid and more nitrogen monoxide (see reaction below), which can be re-oxidized and produce more nitric acid. 3 NO2(g) + H2O(l) → 2 HNO3(aq) + NO(g) Assuming both gases are ...
... D. Cannot determine from information provided. 6. Once nitrogen dioxide is generated, it combines with water to generate nitric acid and more nitrogen monoxide (see reaction below), which can be re-oxidized and produce more nitric acid. 3 NO2(g) + H2O(l) → 2 HNO3(aq) + NO(g) Assuming both gases are ...
Camp 1 - drjosephryan.com Home Page
... The following chemical equation tells us that propane gas and oxygen gas react to form carbon dioxide gas and water vapor C3 H8 ( g) + O2 (g) ...
... The following chemical equation tells us that propane gas and oxygen gas react to form carbon dioxide gas and water vapor C3 H8 ( g) + O2 (g) ...
Chemistry Chapter 2 - Barnstable Academy
... c. contains different kinds of matter b. contains more matter d. has a definite composition ____ 13. An example of an extensive property of matter is ____. a. temperature c. mass b. pressure d. hardness ____ 14. All of the following are physical properties of matter EXCEPT ____. a. mass c. melting p ...
... c. contains different kinds of matter b. contains more matter d. has a definite composition ____ 13. An example of an extensive property of matter is ____. a. temperature c. mass b. pressure d. hardness ____ 14. All of the following are physical properties of matter EXCEPT ____. a. mass c. melting p ...
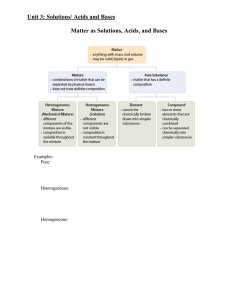
Outline for Unit 1 Solutions, Acid/Base, and Gases
... Strong vs. Weak Bases: Strong base: completely (100%) dissociates into hydroxide (OH-) ions when dissolved in water. All oxides and hydroxides of the alkali metals and alkaline earths – group 1 and 2 are strong bases. (some are not very soluble in water but what does dissolve will ...
... Strong vs. Weak Bases: Strong base: completely (100%) dissociates into hydroxide (OH-) ions when dissolved in water. All oxides and hydroxides of the alkali metals and alkaline earths – group 1 and 2 are strong bases. (some are not very soluble in water but what does dissolve will ...
General Chemistry Questions
... The system is at equilibrium. The system is not at equilibrium; reaction will proceed left to right. The system is not at equilibrium; reaction will proceed right to left. The system cannot reach equilibrium since the ClO3- and Cl- concentrations are not in the stoichiometric ratio. e. There is not ...
... The system is at equilibrium. The system is not at equilibrium; reaction will proceed left to right. The system is not at equilibrium; reaction will proceed right to left. The system cannot reach equilibrium since the ClO3- and Cl- concentrations are not in the stoichiometric ratio. e. There is not ...
Hydrogen Peroxide Formation Rates in a PEMFC Anode and Cathode
... Proton exchange membrane fuel cell 共PEMFC兲 technology, owing to its high efficiency, operational flexibility, and superior modularity, has the capability to be the structural and fundamental unit of an impending hydrogen economy. Two main issues that impede its progress toward commercialization are ...
... Proton exchange membrane fuel cell 共PEMFC兲 technology, owing to its high efficiency, operational flexibility, and superior modularity, has the capability to be the structural and fundamental unit of an impending hydrogen economy. Two main issues that impede its progress toward commercialization are ...
A) I is TRUE, II is FALSE B) I is FALSE, II is TRUE C) I and II
... is exothermic, with an absorption of energy is endothermic, with an absorption of energy can be either exothermic or endothermic ...
... is exothermic, with an absorption of energy is endothermic, with an absorption of energy can be either exothermic or endothermic ...
Chemistry - SchoolNotes.com
... 21) How many grams of chromium are needed to react with an excess of CuSO4 to produce 27 g Cu? 2Cr(s) + 3CuSO4(aq) Cr2(SO4)3(aq) + 3Cu(s) 14.7 g Cr 22) Calcium oxide, or lime, is produced by the thermal decomposition of limestone in the reaction: CaCO3(s) CaO(s) + CO2(g). What mass of lime can b ...
... 21) How many grams of chromium are needed to react with an excess of CuSO4 to produce 27 g Cu? 2Cr(s) + 3CuSO4(aq) Cr2(SO4)3(aq) + 3Cu(s) 14.7 g Cr 22) Calcium oxide, or lime, is produced by the thermal decomposition of limestone in the reaction: CaCO3(s) CaO(s) + CO2(g). What mass of lime can b ...
Supramolecular Assemblies Built from Lanthanide
... interaction with CBs has been investigated;6 further, in their zwitterionic form, they possess a carboxylate group which can be used as a metal complexing site. This heterofunctionality has been used to build chiral one-dimensional assemblies in which lanthanide ions are bound to both L-cysteine and ...
... interaction with CBs has been investigated;6 further, in their zwitterionic form, they possess a carboxylate group which can be used as a metal complexing site. This heterofunctionality has been used to build chiral one-dimensional assemblies in which lanthanide ions are bound to both L-cysteine and ...
Electrons
... ionic compound) if electrons were completely transferred to the more electronegative element. 1. Free elements (uncombined state) have an oxidation number of zero. ...
... ionic compound) if electrons were completely transferred to the more electronegative element. 1. Free elements (uncombined state) have an oxidation number of zero. ...
Solution - Georgetown Independent School District
... H’s are in kJ/MOLE (division applies similarly) Check to ensure that everything cancels out to give you the exact equation you want. Hint** It is often helpful to begin your work backwards from the answer that you want! ...
... H’s are in kJ/MOLE (division applies similarly) Check to ensure that everything cancels out to give you the exact equation you want. Hint** It is often helpful to begin your work backwards from the answer that you want! ...
principles of reactivity: energy and chemical reactions
... Example #1: Given the reaction below, how much heat is produced when 15.0 g of NO2 are produced? ...
... Example #1: Given the reaction below, how much heat is produced when 15.0 g of NO2 are produced? ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.