Physical Chemistry 3: — Chemical Kinetics
... The scriptum gives a summary of the material covered in the scheduled lectures to allow students to repeat the material more economically. It covers basic material that all chemistry students should learn irrespective of their possible inclination towards inorganic, organic or physical chemistry, bu ...
... The scriptum gives a summary of the material covered in the scheduled lectures to allow students to repeat the material more economically. It covers basic material that all chemistry students should learn irrespective of their possible inclination towards inorganic, organic or physical chemistry, bu ...
x - SharpSchool
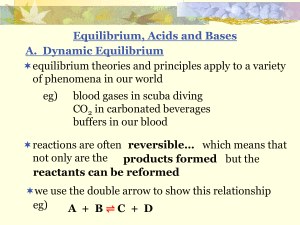
... the stronger an acid, the weaker its conjugate base the weaker an acid, the stronger its conjugate base ...
... the stronger an acid, the weaker its conjugate base the weaker an acid, the stronger its conjugate base ...
Physical Chemistry 3: — Chemical Kinetics - Christian
... textbooks used in the PC-1 and PC-2 courses. This is done in recognition of the established research focus at the Institute of Physical Chemistry at CAU to enable students to pursue B.Sc. thesis projects in Physical Chemistry. Some more specialized sections have been marked by asterisks and may be o ...
... textbooks used in the PC-1 and PC-2 courses. This is done in recognition of the established research focus at the Institute of Physical Chemistry at CAU to enable students to pursue B.Sc. thesis projects in Physical Chemistry. Some more specialized sections have been marked by asterisks and may be o ...
Calculations and the Chemical Equation
... Relating Avogadro's number to molar mass: calculation of the mass of Avogadro's number of sodium atoms Converting moles to atoms. Converting atoms to moles. Converting moles of a substance to mass in grams. Converting kilograms to moles. Converting grams to number of atoms. Calculating formula weigh ...
... Relating Avogadro's number to molar mass: calculation of the mass of Avogadro's number of sodium atoms Converting moles to atoms. Converting atoms to moles. Converting moles of a substance to mass in grams. Converting kilograms to moles. Converting grams to number of atoms. Calculating formula weigh ...
laman web smk raja perempuan, ipoh
... 10. explain the electrochemistry principle in the prevention of rusting and in dental filling 11. describe the importance of the development of better improved batteries for electric cars in terms of smaller size, lower mass and higher voltage. 12. calculate the quantity of product liberated during ...
... 10. explain the electrochemistry principle in the prevention of rusting and in dental filling 11. describe the importance of the development of better improved batteries for electric cars in terms of smaller size, lower mass and higher voltage. 12. calculate the quantity of product liberated during ...
File
... Chemistry 2202 - Unit 1 Test 2 Part 1: For each item, circle the letter corresponding to your choice. ...
... Chemistry 2202 - Unit 1 Test 2 Part 1: For each item, circle the letter corresponding to your choice. ...
National German Competition
... Precipitating zinc ammonium phosphate attention should be paid to the pH value very accurately. The optimal pH value for this precipitation can be adjusted with methyl red (transition to yellow). g) Write down the pH area of transition of methyl red. Which are disturbing side reactions which could o ...
... Precipitating zinc ammonium phosphate attention should be paid to the pH value very accurately. The optimal pH value for this precipitation can be adjusted with methyl red (transition to yellow). g) Write down the pH area of transition of methyl red. Which are disturbing side reactions which could o ...
Stoichiometric Calculations
... Real World Application 2C8H18(g) + 25O2(g) --> 16CO2(g) + 18H2O(g) In your car engine, octane is combusted with oxygen to produce carbon dioxide and water ...
... Real World Application 2C8H18(g) + 25O2(g) --> 16CO2(g) + 18H2O(g) In your car engine, octane is combusted with oxygen to produce carbon dioxide and water ...
Stoichiometric Calculations
... Example: When 10 grams of hydrogen react with 3.4 moles of nitrogen gas to make ammonia, which substance would be the limiting reactant? N2(g) + 3H2(g) --> 2NH3(g) Step 1: Convert all values to moles. 10 g H2 x 1 mol H2 = 5 mol H2 2 g H2 Initial Amounts = 5 mol H2, 3.4 mol N2 ...
... Example: When 10 grams of hydrogen react with 3.4 moles of nitrogen gas to make ammonia, which substance would be the limiting reactant? N2(g) + 3H2(g) --> 2NH3(g) Step 1: Convert all values to moles. 10 g H2 x 1 mol H2 = 5 mol H2 2 g H2 Initial Amounts = 5 mol H2, 3.4 mol N2 ...
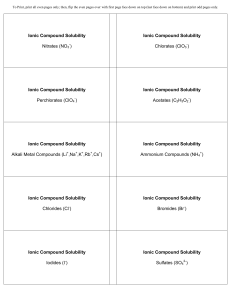
Ionic Compound Solubility Nitrates (NO3 ) Ionic Compound
... Definition A substance that is able to donate a H+ ion (a proton) and, hence, increases the concentration of H+(aq) when it dissolves in water. ...
... Definition A substance that is able to donate a H+ ion (a proton) and, hence, increases the concentration of H+(aq) when it dissolves in water. ...
FREE Sample Here
... 49. In phenylketonuria, an individual cannot break down the amino acid phenylalanine. Molecules that include phenylalanine build up in the blood, which causes intellectual disability and other symptoms. This inherited disease can be controlled by following a diet that is very low in A. carbohydrates ...
... 49. In phenylketonuria, an individual cannot break down the amino acid phenylalanine. Molecules that include phenylalanine build up in the blood, which causes intellectual disability and other symptoms. This inherited disease can be controlled by following a diet that is very low in A. carbohydrates ...
Instructor`s Resource Manual
... are given at the back of the book. Corresponding end-of-chapter Problems are noted at the ends of the Exercises. Problems have been divided into categories: Conceptual Problems, Practice Problems, General Problems, and Cumulative-Skills problems (these have been followed by Media Activities). Answer ...
... are given at the back of the book. Corresponding end-of-chapter Problems are noted at the ends of the Exercises. Problems have been divided into categories: Conceptual Problems, Practice Problems, General Problems, and Cumulative-Skills problems (these have been followed by Media Activities). Answer ...
File
... electron with relative ease to form M+ cations when in ionic compounds. They all are easily oxidized. Therefore, in order to prepare the pure metals, alkali metals must be produced in the absence of materials (H2O, O2) that are capable of oxidizing them. The method of preparation is electrochemical ...
... electron with relative ease to form M+ cations when in ionic compounds. They all are easily oxidized. Therefore, in order to prepare the pure metals, alkali metals must be produced in the absence of materials (H2O, O2) that are capable of oxidizing them. The method of preparation is electrochemical ...
Module 1 Predictor Questions
... Note that this is an example of an ionic compound (see Module 3). The parentheses around (SO4) indicate that it is a polyatomic ion. Its actual formula is SO42-. Two Al3+ ions are required to balance the charge of the three SO42-. So, this formula also tells us that there are 2 Al3+ions and 3 SO42- ...
... Note that this is an example of an ionic compound (see Module 3). The parentheses around (SO4) indicate that it is a polyatomic ion. Its actual formula is SO42-. Two Al3+ ions are required to balance the charge of the three SO42-. So, this formula also tells us that there are 2 Al3+ions and 3 SO42- ...
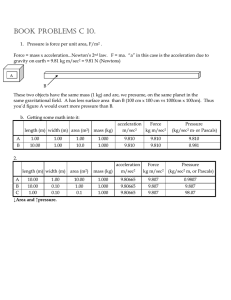
book problems c 10.
... atomic mass, and the unit cell length, determined from x-ray methods. To be useful for this purpose, the crystal must be free of defects. Very accurate values of these quantities for silicon have been measured at the National Institute for Standards and Technology (NIST). To use this approach, it is ...
... atomic mass, and the unit cell length, determined from x-ray methods. To be useful for this purpose, the crystal must be free of defects. Very accurate values of these quantities for silicon have been measured at the National Institute for Standards and Technology (NIST). To use this approach, it is ...
Unit 3 2 Basic Mole Conversions and Mole Maps
... So ... Here's that balanced equation again.... Try to answer the following questions: 2 C2H6(g) + 7 O2(g) ...
... So ... Here's that balanced equation again.... Try to answer the following questions: 2 C2H6(g) + 7 O2(g) ...
Ch. 12 Stoichiometry
... How many molecules of NH3 are needed to produce 2.34 x 1022 molecules of N2F4? How many grams of HF are produced from a reaction of 4.56 x 1023 molecules of F2 with excess NH3? What volume of HF, at STP, can be produced from 345g of NH3? How many molecules of N2F4 can be produce from 45.6L of F2 , a ...
... How many molecules of NH3 are needed to produce 2.34 x 1022 molecules of N2F4? How many grams of HF are produced from a reaction of 4.56 x 1023 molecules of F2 with excess NH3? What volume of HF, at STP, can be produced from 345g of NH3? How many molecules of N2F4 can be produce from 45.6L of F2 , a ...
Support Material
... Explain how vacancies are introduced in a solid NaCl crystal when divalent cations (M2+) are added to molten NaCl. ...
... Explain how vacancies are introduced in a solid NaCl crystal when divalent cations (M2+) are added to molten NaCl. ...
Answers
... The elements nitrogen and fluorine combine to form nitrogen trifluoride, NF3. The balanced chemical equation is N2(g) + 3F2(g) ----------> 2NF3(g) If a sealed reaction vessel contains 1.254 g of N2 and 3.451 g of F2 what mass of NF3 will be formed, assuming complete reaction? Atomic weights: N 14.01 ...
... The elements nitrogen and fluorine combine to form nitrogen trifluoride, NF3. The balanced chemical equation is N2(g) + 3F2(g) ----------> 2NF3(g) If a sealed reaction vessel contains 1.254 g of N2 and 3.451 g of F2 what mass of NF3 will be formed, assuming complete reaction? Atomic weights: N 14.01 ...
1412_lecture_ch16 Fall_2014
... If 2.00 mL of 0.200 M NaOH are added to 1.00 L of 0.100 M CaCl2, will a precipitate form? The ions present in solution are Na+, OH-, Ca2+, Cl-. Only possible precipitate is Ca(OH)2 (solubility rules). Is Q > Ksp for Ca(OH)2? ...
... If 2.00 mL of 0.200 M NaOH are added to 1.00 L of 0.100 M CaCl2, will a precipitate form? The ions present in solution are Na+, OH-, Ca2+, Cl-. Only possible precipitate is Ca(OH)2 (solubility rules). Is Q > Ksp for Ca(OH)2? ...
The Acidic Environment #2
... pollution. This led to regulations to control emissions from factories, power stations and motor cars. The annual average concentration of SO2 and NO2 in most large cities around the world is 0.01 ppm for each gas. This is about 10 times the value for clean air, though a concentration of 0.01 ppm ...
... pollution. This led to regulations to control emissions from factories, power stations and motor cars. The annual average concentration of SO2 and NO2 in most large cities around the world is 0.01 ppm for each gas. This is about 10 times the value for clean air, though a concentration of 0.01 ppm ...
Solving General Chemistry Problems 5e
... only arithmetic and simple algebra. Nevertheless, if you don't understand it, you can expect troubles before long. So, before you can really get into chemistry, you need to master the mathematical operations in the first six chapters. 3. Don't think of your calculator as a security blanket that will ...
... only arithmetic and simple algebra. Nevertheless, if you don't understand it, you can expect troubles before long. So, before you can really get into chemistry, you need to master the mathematical operations in the first six chapters. 3. Don't think of your calculator as a security blanket that will ...
Regents Review Live
... and a strong base: 2 Na (s) + H2O (l) 2 NaOH (aq) + H2 (g) 1 valence electron Form +1 ion by losing that valence electron Form oxides like Na2O, Li2O, K2O ...
... and a strong base: 2 Na (s) + H2O (l) 2 NaOH (aq) + H2 (g) 1 valence electron Form +1 ion by losing that valence electron Form oxides like Na2O, Li2O, K2O ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.