The Role of Medicinal Chemistry in Canadian Pharmacy
... What are the potential Drug interactions? Is it a “Pharmacokinetic” or “Pharmacodynamic” or “Pharmaceutical” drug ...
... What are the potential Drug interactions? Is it a “Pharmacokinetic” or “Pharmacodynamic” or “Pharmaceutical” drug ...
Ch 17 Equilibrium Notes
... 1) 2HI (g) ↔ H2 (g) + I2 (g) A 2.00 L flask is filled with 0.200 mol of HI gas , at equilibrium [HI]= 0.078 M . Calculate Kc. 2) CH4 (g) + H2O (g) ↔ CO (g) + 3H2(g) A 0.32 L flask is filled with methane and water at 1200 K. At equilibrium, the flask contains 0.26 mol of CO, 0.091 mol of H2, 0.01 mol ...
... 1) 2HI (g) ↔ H2 (g) + I2 (g) A 2.00 L flask is filled with 0.200 mol of HI gas , at equilibrium [HI]= 0.078 M . Calculate Kc. 2) CH4 (g) + H2O (g) ↔ CO (g) + 3H2(g) A 0.32 L flask is filled with methane and water at 1200 K. At equilibrium, the flask contains 0.26 mol of CO, 0.091 mol of H2, 0.01 mol ...
word - My eCoach
... When certain ionic solids crystallize from aqueous solutions, a definite number of molecules of water remain attached to the crystal. Ionic solids that contain a definite amount of water are called hydrates or hydrated salts and the water in the crystal structure is called water of hydration. The wa ...
... When certain ionic solids crystallize from aqueous solutions, a definite number of molecules of water remain attached to the crystal. Ionic solids that contain a definite amount of water are called hydrates or hydrated salts and the water in the crystal structure is called water of hydration. The wa ...
Chapter 19 Chemical Thermodynamics
... Enthalpies of Formation An enthalpy of formation, Hf, is defined as the enthalpy change for the reaction in which a compound is made from its constituent elements in their elemental forms. ...
... Enthalpies of Formation An enthalpy of formation, Hf, is defined as the enthalpy change for the reaction in which a compound is made from its constituent elements in their elemental forms. ...
chemical change
... The evidence for a chemical reaction occurring, is the formation of a substance which is different from the original reactant or reactant, this is often accompanied by changes in energy, which are measured as temperature changes. Thus for the reaction of the silver metal sodium with the green/yellow ...
... The evidence for a chemical reaction occurring, is the formation of a substance which is different from the original reactant or reactant, this is often accompanied by changes in energy, which are measured as temperature changes. Thus for the reaction of the silver metal sodium with the green/yellow ...
SCHOOL OF CHEMICAL SCIENCES
... both the teaching and research laboratories. As such, the graduates can pursue career in public and private companies such as the Malaysian Palm Oil Board (MPOB), the Malaysian Agricultural Research and Development Institute (MARDI), the Forestry Research Institute Malaysia (FRIM) and the Chemistry ...
... both the teaching and research laboratories. As such, the graduates can pursue career in public and private companies such as the Malaysian Palm Oil Board (MPOB), the Malaysian Agricultural Research and Development Institute (MARDI), the Forestry Research Institute Malaysia (FRIM) and the Chemistry ...
CH 13
... e) The rate constant for the uncatalyzed reaction at 25C is 5.21 x 10-4/min. The rate constant for the catalyzed reaction at 25C is 2.95 x 108/min. 1) What is the half-life of the uncatalyzed reaction at 25C? 2) What is the half-life of the catalyzed reaction? ...
... e) The rate constant for the uncatalyzed reaction at 25C is 5.21 x 10-4/min. The rate constant for the catalyzed reaction at 25C is 2.95 x 108/min. 1) What is the half-life of the uncatalyzed reaction at 25C? 2) What is the half-life of the catalyzed reaction? ...
4 Expressing and Measuring Chemical Change
... occurs remains constant. Priestley and Lavoisier were both radical thinkers for the time they lived in. Englishman Priestley’s support of the French Revolutionists led to his persecution, and he eventually had to flee from Europe altogether. He lived the last of his days in Pennsylvania in the Unite ...
... occurs remains constant. Priestley and Lavoisier were both radical thinkers for the time they lived in. Englishman Priestley’s support of the French Revolutionists led to his persecution, and he eventually had to flee from Europe altogether. He lived the last of his days in Pennsylvania in the Unite ...
L-11 Chemical thermodynamics
... there would be no exchange of matter or energy between the system and the surroundings. We call such a system an isolated system. Isolated system is a system which can exchange neither matter nor energy with the surroundings. If we keep hot tea/milk in a stoppered stainless steel flask, it will not ...
... there would be no exchange of matter or energy between the system and the surroundings. We call such a system an isolated system. Isolated system is a system which can exchange neither matter nor energy with the surroundings. If we keep hot tea/milk in a stoppered stainless steel flask, it will not ...
CHAPTER TWO SOLID STATE REACTIONS 2.0 Introduction The
... Reactions between or within solid reactants to yield a solid product are prototypical of solvent free reactions [3, 4]. From literature reports the concept of chemical reactivity between solids (and very often within solids) is very difficult to define [5]. Reactions which occurred in the melt were ...
... Reactions between or within solid reactants to yield a solid product are prototypical of solvent free reactions [3, 4]. From literature reports the concept of chemical reactivity between solids (and very often within solids) is very difficult to define [5]. Reactions which occurred in the melt were ...
High School Knowledge Exam – Study Guide
... -A physical change is a change in which no new substance is formed (no new molecules formed) -A chemical change results in the formation of one or more new substances (new molecules) Physical Change examples: Phase change, physically altering something (cutting it up, etc.), dissolving salt in water ...
... -A physical change is a change in which no new substance is formed (no new molecules formed) -A chemical change results in the formation of one or more new substances (new molecules) Physical Change examples: Phase change, physically altering something (cutting it up, etc.), dissolving salt in water ...
1st-Year-ch-wise-test
... Test : Chemistry Time Allowed : 20 min F.Sc : Part I Max. Marks : 15 Q. No.2: Give short answers to the followings. ...
... Test : Chemistry Time Allowed : 20 min F.Sc : Part I Max. Marks : 15 Q. No.2: Give short answers to the followings. ...
IFS_Question_Paper_Chemical_engineering
... (a) Find the first-order rate constant for the disappearance of A in the gaseous reaction 2A → R, holding the pressure constant, the volume of the reaction mixture starting with 80 per cent A decreases by 20 per cent in 3 mm. ...
... (a) Find the first-order rate constant for the disappearance of A in the gaseous reaction 2A → R, holding the pressure constant, the volume of the reaction mixture starting with 80 per cent A decreases by 20 per cent in 3 mm. ...
Chapter 3
... • For each mole of CO2, there is one mole of C. Convert moles of C to grams of C. • 0.561 g CO2 x (1 mol CO2 / 44.01 g CO2) x (1 mol C / 1 mol CO2) (12.01 g C/ mol C) = 0.153 g C ...
... • For each mole of CO2, there is one mole of C. Convert moles of C to grams of C. • 0.561 g CO2 x (1 mol CO2 / 44.01 g CO2) x (1 mol C / 1 mol CO2) (12.01 g C/ mol C) = 0.153 g C ...
Types of Chemical Reactions
... Background: atomic masses Look at the “atomic masses” on the periodic table. What do these represent? E.g. the atomic mass of C is 12 (atomic # is 6) We know there are 6 protons and 6 neutrons Protons and neutrons have roughly the same mass. So, C weighs 12 u (atomic mass units). What is th ...
... Background: atomic masses Look at the “atomic masses” on the periodic table. What do these represent? E.g. the atomic mass of C is 12 (atomic # is 6) We know there are 6 protons and 6 neutrons Protons and neutrons have roughly the same mass. So, C weighs 12 u (atomic mass units). What is th ...
KEY
... 4. decreases Explanation: Increasing the total pressure on the system by decreasing its volume will shift the equilibrium toward the side of the reaction with fewer numbers of moles of gaseous components. If the total number of moles of gas is the same on the product and reactant sides of the balanc ...
... 4. decreases Explanation: Increasing the total pressure on the system by decreasing its volume will shift the equilibrium toward the side of the reaction with fewer numbers of moles of gaseous components. If the total number of moles of gas is the same on the product and reactant sides of the balanc ...
Chemical Dynamics at Surfaces
... Irving Langmuir was born in Brooklyn, New York, on January 31, 1881, as the third of four sons of Charles Langmuir and Sadie, neé Comings. His early education was obtained in various schools and institutes in the USA, and in Paris (1892-1895). He graduated as a metallurgical engineer from the School ...
... Irving Langmuir was born in Brooklyn, New York, on January 31, 1881, as the third of four sons of Charles Langmuir and Sadie, neé Comings. His early education was obtained in various schools and institutes in the USA, and in Paris (1892-1895). He graduated as a metallurgical engineer from the School ...
ROCZNIKI FILOZOFICZNE Tom XXXI, zeszyt 3 — 1983
... (see for example 1, 21). These successful attempts proved J. Bernal's hypothesis that aluminosilicates took part in the synthesis of biologically important compounds to be reasonable. Aluminosilicates may play the part of adsorbents and catalysts and provide matrices for synthetized organic structur ...
... (see for example 1, 21). These successful attempts proved J. Bernal's hypothesis that aluminosilicates took part in the synthesis of biologically important compounds to be reasonable. Aluminosilicates may play the part of adsorbents and catalysts and provide matrices for synthetized organic structur ...
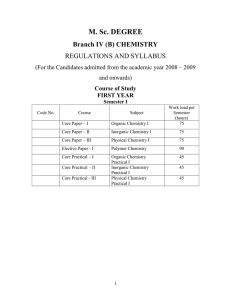
M.Sc. Chemistry - Periyar University
... Effect of structure on reactivity – resonance and fields effects, steric effects, quantitative treatment – the Hammett equation and linear free energy relationship, substituent and reaction constant, Taft equation. Thermodynamic and kinetic requirements for reactions, thermodynamic and kinetic contr ...
... Effect of structure on reactivity – resonance and fields effects, steric effects, quantitative treatment – the Hammett equation and linear free energy relationship, substituent and reaction constant, Taft equation. Thermodynamic and kinetic requirements for reactions, thermodynamic and kinetic contr ...
Plasma Clean to Reduce Wire Bond Failures
... provide insights into what is on the surface. This information can also be useful in deciding which plasma process to use. Neither technique, however, is practicable for production QC and both are very expensive to operate. In addition, today’s contaminant may not be tomorrow’s contaminant so any QC ...
... provide insights into what is on the surface. This information can also be useful in deciding which plasma process to use. Neither technique, however, is practicable for production QC and both are very expensive to operate. In addition, today’s contaminant may not be tomorrow’s contaminant so any QC ...
Grade 11 Review Package
... substances can be further classified into elements and compounds. • An element is a pure substance that cannot be separated chemically into any simpler substances. Oxygen gas, O2(g), solid carbon, C(s) , and copper metal, Cu(s) , are examples of elements. • A compound is a pure substance that result ...
... substances can be further classified into elements and compounds. • An element is a pure substance that cannot be separated chemically into any simpler substances. Oxygen gas, O2(g), solid carbon, C(s) , and copper metal, Cu(s) , are examples of elements. • A compound is a pure substance that result ...