KEY CONCEPT Enzymes are catalysts for chemical
... A catalyst lowers activation energy. • Catalysts are substances that speed up chemical reactions. – decrease activation energy – increase reaction rate ...
... A catalyst lowers activation energy. • Catalysts are substances that speed up chemical reactions. – decrease activation energy – increase reaction rate ...
Dr Davids Essential Chemistry Definitions Bk1
... The number of particles present in 1 mole of a substance. It has a numerical value of 6.02 x 1023 mol-1 Oxidation number: The difference between the number of electrons associated with an element in a compound and the element itself. Just for the purpose of assigning oxidation numbers all compounds ...
... The number of particles present in 1 mole of a substance. It has a numerical value of 6.02 x 1023 mol-1 Oxidation number: The difference between the number of electrons associated with an element in a compound and the element itself. Just for the purpose of assigning oxidation numbers all compounds ...
Chapter 4 - U of L Class Index
... just masses). This is why it is essential to have balanced reaction equations*** ...
... just masses). This is why it is essential to have balanced reaction equations*** ...
Chemical Reactions
... What is a chemical reaction? • A chemical reaction is the process by which the atoms of one or more substances are rearranged to form different substances. ...
... What is a chemical reaction? • A chemical reaction is the process by which the atoms of one or more substances are rearranged to form different substances. ...
CHM 2045C - State College of Florida
... (5 Credit Hours) (A.A.) Three hours lecture, three hours laboratory per week. Prerequisites: Completion of MAC 1105. Completion of CHM 1025C with a grade of “C” or better or one year of high school college preparatory honors or AP chemistry within last three years. This course meets Area V for the A ...
... (5 Credit Hours) (A.A.) Three hours lecture, three hours laboratory per week. Prerequisites: Completion of MAC 1105. Completion of CHM 1025C with a grade of “C” or better or one year of high school college preparatory honors or AP chemistry within last three years. This course meets Area V for the A ...
Name - rwebbchem
... with AgNO3, Pb(NO3)2, and BaCl2. Precipitates form in all three cases. Which of the following could be the anion of the unknown salt: Br-, CO32-, or NO3-? ...
... with AgNO3, Pb(NO3)2, and BaCl2. Precipitates form in all three cases. Which of the following could be the anion of the unknown salt: Br-, CO32-, or NO3-? ...
Chapter 8: Chemical Reactions and Physical Changes
... • Mass number: total protons and neutrons in an atom’s nucleus • Atomic mass: the average mass of a sample of atoms of that element found in nature • Periodic table: chart that arranges elements by atomic number into rows and columns according to similarities in their properties ...
... • Mass number: total protons and neutrons in an atom’s nucleus • Atomic mass: the average mass of a sample of atoms of that element found in nature • Periodic table: chart that arranges elements by atomic number into rows and columns according to similarities in their properties ...
Chapter 14 Chemical Reactions
... 14.1 Balancing chemical equations A balanced chemical equation has the same number of each type of atom on the product side and the reactant side. To balance the equation, we add another water molecule to the product side and add another oxygen molecule to the reactant side. ...
... 14.1 Balancing chemical equations A balanced chemical equation has the same number of each type of atom on the product side and the reactant side. To balance the equation, we add another water molecule to the product side and add another oxygen molecule to the reactant side. ...
Types of Chemical Reactions
... of a combination of carbon and hydrogen) to form water and carbon dioxide. These reactions are exothermic, meaning they produce heat. This reaction can be expressed as: ...
... of a combination of carbon and hydrogen) to form water and carbon dioxide. These reactions are exothermic, meaning they produce heat. This reaction can be expressed as: ...
Reaction Analysis and PAT Tools
... iC IR™ software was designed to take infrared data and convert it into useful and meaningful information about chemical reactions, in real time. The result of an extensive research project on how scientists analyze reactions, iC IR allows chemists and engineers to quickly gain an understanding of th ...
... iC IR™ software was designed to take infrared data and convert it into useful and meaningful information about chemical reactions, in real time. The result of an extensive research project on how scientists analyze reactions, iC IR allows chemists and engineers to quickly gain an understanding of th ...
Notes for Types of Reactions:
... chemical reaction = the _________ by which one or more substances are __________ into one or more _________ substances. in any chemical reaction, the _________ substances are known as the reactants and the __________ substances are known as the products. total mass of reactants = according to ...
... chemical reaction = the _________ by which one or more substances are __________ into one or more _________ substances. in any chemical reaction, the _________ substances are known as the reactants and the __________ substances are known as the products. total mass of reactants = according to ...
Nuclear Astrophysics (1)
... Explosive Burning above a critical temperature destroys (photodisintegrates) all nuclei and (re)builds them up during the expansion. Dependent on density, the full NSE is maintained and leads to only Fegroup nuclei (normal freezeout) or the reactions linking 4He to C and be ...
... Explosive Burning above a critical temperature destroys (photodisintegrates) all nuclei and (re)builds them up during the expansion. Dependent on density, the full NSE is maintained and leads to only Fegroup nuclei (normal freezeout) or the reactions linking 4He to C and be ...
Chemistry Semester Test Study Guide Chapters
... What state of matter has a definite volume and takes the shape of its container? Which state of matter takes both the shape and volume of its container? In a chemical reaction, what are the reactants and what are the products? If the total mass of the reactants in a chemical reaction is 60 g, what i ...
... What state of matter has a definite volume and takes the shape of its container? Which state of matter takes both the shape and volume of its container? In a chemical reaction, what are the reactants and what are the products? If the total mass of the reactants in a chemical reaction is 60 g, what i ...
PowerPoint
... the CO conversion if the temperature is (a) 150 °C, (b) 250 °C and (c) 350 °C? ‣ Noting that the water-gas shift reaction is exothermic; predict whether the equilibrium conversion will increase or decrease as the temperature increases before you perform the calculations ...
... the CO conversion if the temperature is (a) 150 °C, (b) 250 °C and (c) 350 °C? ‣ Noting that the water-gas shift reaction is exothermic; predict whether the equilibrium conversion will increase or decrease as the temperature increases before you perform the calculations ...
Equation Intro Worksheet 1213
... Look at the above picture and the ones on pages 325-327 to see why these reactions are drawn the way they are…(note that the book uses colors to identify each element’s atoms where I’ve used letters because this is a black and white photocopy) 5. In the space below, draw the reaction written…use num ...
... Look at the above picture and the ones on pages 325-327 to see why these reactions are drawn the way they are…(note that the book uses colors to identify each element’s atoms where I’ve used letters because this is a black and white photocopy) 5. In the space below, draw the reaction written…use num ...
Review Sheet: Unit 6 Name__________________ CHEMISTRY: A
... reactants are two or more ____________ and/or compounds and a more ____________ product is formed. A ____________ reaction is just the opposite; a single compound is broken down into two or more simpler substances. In a ____________ ____________ reaction, the reactants and products take the general ...
... reactants are two or more ____________ and/or compounds and a more ____________ product is formed. A ____________ reaction is just the opposite; a single compound is broken down into two or more simpler substances. In a ____________ ____________ reaction, the reactants and products take the general ...
Name
... 21. In what units is molar mass typically expressed? a. Kg b. L c. Amu d. g/mol 22. The sum of the percentages in the percentage composition of a substance equals a. 100. b. the molar mass. c. the molar volume. d. Avogadro’s number. 23. The simplest whole-number ratio of the atoms of the elements in ...
... 21. In what units is molar mass typically expressed? a. Kg b. L c. Amu d. g/mol 22. The sum of the percentages in the percentage composition of a substance equals a. 100. b. the molar mass. c. the molar volume. d. Avogadro’s number. 23. The simplest whole-number ratio of the atoms of the elements in ...
Classifying Chemical Reactions 9-3
... We need one more oxygen in the products. Can’t change the formula, because it describes what it is (carbon monoxide in this example) ...
... We need one more oxygen in the products. Can’t change the formula, because it describes what it is (carbon monoxide in this example) ...
Chem 400 Chem 150 REVIEW SHEET Amanda R
... Atoms, Molecules, Ions – fundamentals of elements o Protons, electrons and neutrons make up an atom o Atoms make up molecules, all matter is made of atoms o Protons and neutrons are in the nucleus, and electrons are buzzing outside the nucleus around the nucleus in orbitals o # of protons defines an ...
... Atoms, Molecules, Ions – fundamentals of elements o Protons, electrons and neutrons make up an atom o Atoms make up molecules, all matter is made of atoms o Protons and neutrons are in the nucleus, and electrons are buzzing outside the nucleus around the nucleus in orbitals o # of protons defines an ...
PRACTICE * Naming and Writing Ionic Compounds
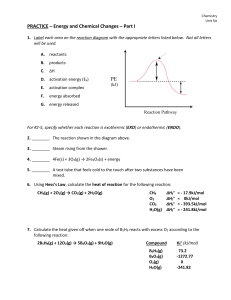
... 1. Label each area on the reaction diagram with the appropriate letters listed below. Not all letters will be used. A. ...
... 1. Label each area on the reaction diagram with the appropriate letters listed below. Not all letters will be used. A. ...