C H A P T E R
... number of particles. To make counting such large numbers easier, scientists use the same approach to represent the number of ions or molecules in a sample as they use for atoms. The SI unit for amount is called the mole (mol). A mole is the number of atoms in exactly 12 grams of carbon-12. The numbe ...
... number of particles. To make counting such large numbers easier, scientists use the same approach to represent the number of ions or molecules in a sample as they use for atoms. The SI unit for amount is called the mole (mol). A mole is the number of atoms in exactly 12 grams of carbon-12. The numbe ...
Clays form a class of technologically important - Eu
... EDS compositional studies have been carried out on bulk and on grains of the samples. For the characterization of the bulk ten measurements, taking care to avoid the iron-rich grains, were performed scanning different areas with dimensions about 800x800 m2. Then the results were obtained using the ...
... EDS compositional studies have been carried out on bulk and on grains of the samples. For the characterization of the bulk ten measurements, taking care to avoid the iron-rich grains, were performed scanning different areas with dimensions about 800x800 m2. Then the results were obtained using the ...
Pentose P Path
... Transketolase & Transaldolase catalyze transfer of 2-C or 3-C molecular fragments respectively, in each case from a ketose donor to an aldose acceptor. D. E. Nicholson has suggested that the names of these enzymes should be changed, since Transketolase actually transfers an aldol moiety (glycoald ...
... Transketolase & Transaldolase catalyze transfer of 2-C or 3-C molecular fragments respectively, in each case from a ketose donor to an aldose acceptor. D. E. Nicholson has suggested that the names of these enzymes should be changed, since Transketolase actually transfers an aldol moiety (glycoald ...
Small Glycosylated Lignin Oligomers Are Stored in
... The purified vacuoles were then subjected to phenolic profiling using ultra-high-performance liquid chromatography (UHPLC)electrospray ionization-Fourier transform-ion cyclotron resonancemass spectrometry in negative ionization mode (Figure 3A) to identify as many peaks as possible associated with (ne ...
... The purified vacuoles were then subjected to phenolic profiling using ultra-high-performance liquid chromatography (UHPLC)electrospray ionization-Fourier transform-ion cyclotron resonancemass spectrometry in negative ionization mode (Figure 3A) to identify as many peaks as possible associated with (ne ...
Exam Review
... At various levels in the tower, trays collect mixtures of substances as they condense, each mixture containing compounds with similar boiling points. These mixtures are called petroleum fractions. The fractions with the lowest boiling points contain the smallest molecules. The low boiling points are ...
... At various levels in the tower, trays collect mixtures of substances as they condense, each mixture containing compounds with similar boiling points. These mixtures are called petroleum fractions. The fractions with the lowest boiling points contain the smallest molecules. The low boiling points are ...
synthesis and reactions of tris dialkyl dithiocarbamates of group 15
... centrosymmetric halogen–bridged dimer. This demonstrates obviously the risk of structural elucidation from spectroscopic data alone. Correlations between solid state structure and vibrational spectra have been applied to novel 1:1 adducts like HgX2(PR3) (where R3 = ph2Me, phMe2, Et3, Me3 and But3; X ...
... centrosymmetric halogen–bridged dimer. This demonstrates obviously the risk of structural elucidation from spectroscopic data alone. Correlations between solid state structure and vibrational spectra have been applied to novel 1:1 adducts like HgX2(PR3) (where R3 = ph2Me, phMe2, Et3, Me3 and But3; X ...
Supporting Information - Royal Society of Chemistry
... absorbance followed at 450 nm). The kinetic data were analyzed via the double reciprocal plots of the initial rates of the enzyme catalyses and the substrate concentrations in the presence of different concentrations of inhibitors. For clarity, such plots are shown only for selected concentrations o ...
... absorbance followed at 450 nm). The kinetic data were analyzed via the double reciprocal plots of the initial rates of the enzyme catalyses and the substrate concentrations in the presence of different concentrations of inhibitors. For clarity, such plots are shown only for selected concentrations o ...
Chapter 3: Mass Relationships in Chemical
... 58. A mass spectrometer works by ionizing atoms or molecules, and then accelerating them past oppositely charged plates. The mass is obtained by A) measuring the force of impact on a detecting screen, and then calculating the mass using force = mass acceleration. B) suspending the ions in an appli ...
... 58. A mass spectrometer works by ionizing atoms or molecules, and then accelerating them past oppositely charged plates. The mass is obtained by A) measuring the force of impact on a detecting screen, and then calculating the mass using force = mass acceleration. B) suspending the ions in an appli ...
Mammalian Cell Culture: High Throughput Applications of
... The history of cell culture dates back to early twentieth century. However, it was only during the 1940’s and 1950’s that there was a rapid development in the techniques for cell culture. Mammalian cells are cells which are generally part of an organ of an organism, differentiated to perform specifi ...
... The history of cell culture dates back to early twentieth century. However, it was only during the 1940’s and 1950’s that there was a rapid development in the techniques for cell culture. Mammalian cells are cells which are generally part of an organ of an organism, differentiated to perform specifi ...
Effect of fluoro-polycarbonates containing aliphatic
... and 19F NMR spectra were obtained via a Bruker AVANCE NMR spectrometer, operated at 500, 125, or 300 MHz, respectively. ...
... and 19F NMR spectra were obtained via a Bruker AVANCE NMR spectrometer, operated at 500, 125, or 300 MHz, respectively. ...
Sample Exercise 2.1
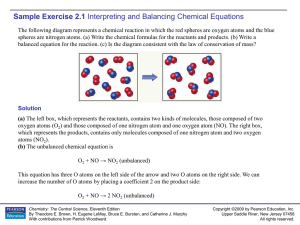
... Now there are two N atoms and four O atoms on the right. Placing the coefficient 2 in front of NO balances both the number of N atoms and O atoms: O2 + 2 NO → 2 NO2 (balanced) (c) The left box (reactants) contains four O2 molecules and eight NO molecules. Thus, the molecular ratio is one O2 for each ...
... Now there are two N atoms and four O atoms on the right. Placing the coefficient 2 in front of NO balances both the number of N atoms and O atoms: O2 + 2 NO → 2 NO2 (balanced) (c) The left box (reactants) contains four O2 molecules and eight NO molecules. Thus, the molecular ratio is one O2 for each ...
F:\Users\Steven\Documents\Chemistry\CHEM120\Problem Set
... b) Calculate the final concentration of the silver after all the precipitate (solid) has formed. 2) When 75 mL of 0.20M Na3PO4 is added to 125 mL of 0.30 M Zn(NO3)2 a white solid forms. a) Please write the NET ionic reaction that occurred. b) How many grams of solid were made? c) What is the concent ...
... b) Calculate the final concentration of the silver after all the precipitate (solid) has formed. 2) When 75 mL of 0.20M Na3PO4 is added to 125 mL of 0.30 M Zn(NO3)2 a white solid forms. a) Please write the NET ionic reaction that occurred. b) How many grams of solid were made? c) What is the concent ...
13C MRS: An outstanding tool for metabolic studies
... ability of 13C MRS to (i) perform repetitive, noninvasive measurements of metabolic processes as they proceed in their own intracellular environment and (ii) its capacity to measure unique physical properties not detectable by other methodologies, such as spin coupling patterns, isotopic shifts, or ...
... ability of 13C MRS to (i) perform repetitive, noninvasive measurements of metabolic processes as they proceed in their own intracellular environment and (ii) its capacity to measure unique physical properties not detectable by other methodologies, such as spin coupling patterns, isotopic shifts, or ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... 4. The lowest number of moles becomes the divisor for the others. (gives a mole ratio greater than 1) 5. Adjust mole ratios so all numbers are whole (1, 2, etc) 6. The lowest whole-number ratio of moles is the ...
... 4. The lowest number of moles becomes the divisor for the others. (gives a mole ratio greater than 1) 5. Adjust mole ratios so all numbers are whole (1, 2, etc) 6. The lowest whole-number ratio of moles is the ...
BASIC CONCEPTS OF CHEMISTRY
... formation , and is denoted H0. The standard heat of formation of simple substance in its most stable modification shall be equal to zero. Calculation of the heat of the reaction from the heats of formation of the participating substances, is produced by the Hess’ law , the heat of the chemical reac ...
... formation , and is denoted H0. The standard heat of formation of simple substance in its most stable modification shall be equal to zero. Calculation of the heat of the reaction from the heats of formation of the participating substances, is produced by the Hess’ law , the heat of the chemical reac ...
9/10/10 1 Chemistry 121: Atomic and Molecular Chemistry
... Chemistry 121: Atomic and Molecular Chemistry Topic 2: Stoichiometry and Related Atomic Number, Mass Number, and Isotopes • Atoms can be identified by the number of protons and neutrons they contain. • The atomic number (Z) is the number of protons in the nucleus of each atom of an element. • In ...
... Chemistry 121: Atomic and Molecular Chemistry Topic 2: Stoichiometry and Related Atomic Number, Mass Number, and Isotopes • Atoms can be identified by the number of protons and neutrons they contain. • The atomic number (Z) is the number of protons in the nucleus of each atom of an element. • In ...
The biological synthesis of cholesterol
... once the tenacyclic ring system is established, two hydrogen atoms and two methyl groups migrate to neighboring positions in a concerted rearrangement that terminates in lanosterol. Submitting these postulates to experimental test we obtained results which were in each instance in accordance with th ...
... once the tenacyclic ring system is established, two hydrogen atoms and two methyl groups migrate to neighboring positions in a concerted rearrangement that terminates in lanosterol. Submitting these postulates to experimental test we obtained results which were in each instance in accordance with th ...
Exam 1
... Marks will not be deducted for incorrect answers. No marks will be given if more than one answer is completed for any question. ...
... Marks will not be deducted for incorrect answers. No marks will be given if more than one answer is completed for any question. ...
Stoichiometry
... all the reactants to decide which reactant mixture makes the most product. We must always think about how much product can be formed by using what we are given, and the ratio in the balanced equation. ...
... all the reactants to decide which reactant mixture makes the most product. We must always think about how much product can be formed by using what we are given, and the ratio in the balanced equation. ...
odd - WWW2
... but (x + y) = 5, the sum of the iron ions. Hence by substitution, x = 3 and y = 2. Thus there are three Fe2+ ions and two Fe3+ ions per formula. 14.29 Zeolites are used as ion exchangers for water; as adsorption agents, particularly for water in organic solvents; for gas separation, particularly dio ...
... but (x + y) = 5, the sum of the iron ions. Hence by substitution, x = 3 and y = 2. Thus there are three Fe2+ ions and two Fe3+ ions per formula. 14.29 Zeolites are used as ion exchangers for water; as adsorption agents, particularly for water in organic solvents; for gas separation, particularly dio ...
Physiology of the thermophilic acetogen Moorella - The Keep
... Acetogens have been thought of as being metabolically limited and thermodynamically disadvantaged, but just the opposite is true. They can utilize a wide variety of electron donors and electron acceptors, and ...
... Acetogens have been thought of as being metabolically limited and thermodynamically disadvantaged, but just the opposite is true. They can utilize a wide variety of electron donors and electron acceptors, and ...
Systematic metabolic analysis of recombinant Pichia pastoris UNIVERSITAT AUTÒNOMA DE BARCELONA
... Nutrients are transported through the cell membrane using different mechanism in order to be available to be catabolised. Once there are inside, these nutrients are the precursors to generate all the energy and reducing power to synthesize all the cell components required. The metabolic pathways ens ...
... Nutrients are transported through the cell membrane using different mechanism in order to be available to be catabolised. Once there are inside, these nutrients are the precursors to generate all the energy and reducing power to synthesize all the cell components required. The metabolic pathways ens ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.