Structure and Sodium Ion Dynamics in Sodium
... similar approach used previously on Na2O·2SiO2 glass around 635− 699 °C.23 Spin−lattice relaxation times T1ρ in the rotating frame were obtained under static condition with a spin lock sequence at frequencies of ν1(23Na) ≈ 20 and 35 kHz on the 4 mm HX MAS high temperature NMR probe and at ≈ 6.7 and ...
... similar approach used previously on Na2O·2SiO2 glass around 635− 699 °C.23 Spin−lattice relaxation times T1ρ in the rotating frame were obtained under static condition with a spin lock sequence at frequencies of ν1(23Na) ≈ 20 and 35 kHz on the 4 mm HX MAS high temperature NMR probe and at ≈ 6.7 and ...
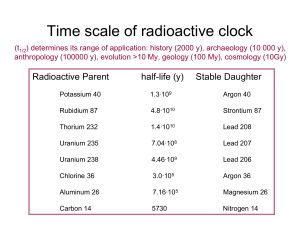
Carbon Dating Method
... the source of the activity. They only can operate successfully if it is assured that the detected event actually has originated in the 14C decay. That requires background suppression techniques. The main background is originated from the cosmic rays and natural radioactivity contained in the surroun ...
... the source of the activity. They only can operate successfully if it is assured that the detected event actually has originated in the 14C decay. That requires background suppression techniques. The main background is originated from the cosmic rays and natural radioactivity contained in the surroun ...
Chemical Reaction Equations
... In order to make sure this happens, more of the other reactant must be present than is required An excess reagent is the reactant whose entities are present in surplus amounts, so that some remain after the reaction ends.. In our reaction: much more copper was used than needed (evidenced by the unre ...
... In order to make sure this happens, more of the other reactant must be present than is required An excess reagent is the reactant whose entities are present in surplus amounts, so that some remain after the reaction ends.. In our reaction: much more copper was used than needed (evidenced by the unre ...
Chapter 23 + Practice Problems - Bloomsburg Area School District
... animals cannot make all of their own carbohydrates, they must get them from food. Carbohydrates provide nearly all of the energy that is available in most plant-derived food. ...
... animals cannot make all of their own carbohydrates, they must get them from food. Carbohydrates provide nearly all of the energy that is available in most plant-derived food. ...
Chapter 4 Alcohols and Alkyl Halides
... Boiling Point. When describing the effect of alkane structure on boiling point in Section 2.14, we pointed out that the forces of attraction between neutral molecules are of three types listed here. The first two of these involve induced dipoles and are often referred to as dispersion forces, or Lon ...
... Boiling Point. When describing the effect of alkane structure on boiling point in Section 2.14, we pointed out that the forces of attraction between neutral molecules are of three types listed here. The first two of these involve induced dipoles and are often referred to as dispersion forces, or Lon ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... water and the solvent pulls the individual ions from the crystal and solvates them. Recall: Ionic substances contain a metal & nonmetal or polyatomic ion. Examples: NH4Cl or KBr Aqueous Reactions © 2009, Prentice-Hall, Inc. ...
... water and the solvent pulls the individual ions from the crystal and solvates them. Recall: Ionic substances contain a metal & nonmetal or polyatomic ion. Examples: NH4Cl or KBr Aqueous Reactions © 2009, Prentice-Hall, Inc. ...
Theoretical Approaches to the Evolutionary Optimization of Glycolysis
... The thermodynamic analysis developed in [14] led to a surprising result relating kinetic and thermodynamic features ; in a metabolic system under a given total affinity (a net exergonism), the different distributions of the local exergonisms produce great differences in the kinetic efficiency of the ...
... The thermodynamic analysis developed in [14] led to a surprising result relating kinetic and thermodynamic features ; in a metabolic system under a given total affinity (a net exergonism), the different distributions of the local exergonisms produce great differences in the kinetic efficiency of the ...
Unit 10 complete 2016-2017
... Moles of A to Moles of B Work the following out on a separate sheet of paper. 1. Hydrogen and oxygen react under certain conditions to product water. a. How many moles of hydrogen would be needed to produce 5.0 moles of water? b. How many moles of oxygen would be needed to produce 5.0 moles of water ...
... Moles of A to Moles of B Work the following out on a separate sheet of paper. 1. Hydrogen and oxygen react under certain conditions to product water. a. How many moles of hydrogen would be needed to produce 5.0 moles of water? b. How many moles of oxygen would be needed to produce 5.0 moles of water ...
Final Exam
... ____ 27. The osmotic pressure of blood is 7.65 atm at 37 C. What mass of glucose (C6H12O6, molar mass = 180.2 g/mol) is needed to prepare 5.00 L of solution for intravenous injection? The osmotic pressure of the glucose solution must equal the osmotic pressure of blood. (R = 0.08206 L·atm/mol·K) a. ...
... ____ 27. The osmotic pressure of blood is 7.65 atm at 37 C. What mass of glucose (C6H12O6, molar mass = 180.2 g/mol) is needed to prepare 5.00 L of solution for intravenous injection? The osmotic pressure of the glucose solution must equal the osmotic pressure of blood. (R = 0.08206 L·atm/mol·K) a. ...
Honors Chemistry
... Moles of A to Moles of B Work the following out on a separate sheet of paper. 1. Hydrogen and oxygen react under certain conditions to product water. a. How many moles of hydrogen would be needed to produce 5.0 moles of water? b. How many moles of oxygen would be needed to produce 5.0 moles of water ...
... Moles of A to Moles of B Work the following out on a separate sheet of paper. 1. Hydrogen and oxygen react under certain conditions to product water. a. How many moles of hydrogen would be needed to produce 5.0 moles of water? b. How many moles of oxygen would be needed to produce 5.0 moles of water ...
A high-field solid-state Cl NMR and quantum chemical
... A series of six L-amino acid hydrochloride salts has been studied by 35/37Cl solid-state NMR spectroscopy (at 11.75 and 21.1 T) and complementary quantum chemical calculations. Analyses of NMR spectra acquired under static and magic-angle-spinning conditions for the six hydrochloride salts, those of ...
... A series of six L-amino acid hydrochloride salts has been studied by 35/37Cl solid-state NMR spectroscopy (at 11.75 and 21.1 T) and complementary quantum chemical calculations. Analyses of NMR spectra acquired under static and magic-angle-spinning conditions for the six hydrochloride salts, those of ...
Ph.D. Thesis --
... observation of carbon nanotubes. Their interesting electronic and mechanical properties make the nanotubes ideal candidates not only for basic but also for applied science. In the last decade, numerous ordered carbon nanostructures were discovered including carbon onions,4 crossed nanotubes,5 peapod ...
... observation of carbon nanotubes. Their interesting electronic and mechanical properties make the nanotubes ideal candidates not only for basic but also for applied science. In the last decade, numerous ordered carbon nanostructures were discovered including carbon onions,4 crossed nanotubes,5 peapod ...
PDF
... essential roles in global carbon, nitrogen, and sulfur cycles, as many of them are known to assimilate nitrogen and/or oxidize sulfide in conjunction with their central carbon and energy metabolisms. Inorganic and organic carbon sources are used for building cellular building blocks of photoautotroph ...
... essential roles in global carbon, nitrogen, and sulfur cycles, as many of them are known to assimilate nitrogen and/or oxidize sulfide in conjunction with their central carbon and energy metabolisms. Inorganic and organic carbon sources are used for building cellular building blocks of photoautotroph ...
Chapter One Hemilabile Ligands in Transition
... protonation at the terminal nitrogen and nucleophilic attack at the vicinal nitrogen.1 The free ligand, N2, is of course, notably unreactive. ...
... protonation at the terminal nitrogen and nucleophilic attack at the vicinal nitrogen.1 The free ligand, N2, is of course, notably unreactive. ...
Assessing the Potential for the Reactions of
... base-catalyzed process), while the logarithm of the pseudo-firstorder rate constant for the amine addition product stays relatively constant. (The slight increase in the amine−epoxide reaction pseudo-first-order rate constants at high pH is most likely not due to the role of base catalysis but rather ...
... base-catalyzed process), while the logarithm of the pseudo-firstorder rate constant for the amine addition product stays relatively constant. (The slight increase in the amine−epoxide reaction pseudo-first-order rate constants at high pH is most likely not due to the role of base catalysis but rather ...
lithium
... lithium-6 (7.5 percent); five radioactive isotopes have been prepared—lithium-5, lithium-8, lithium-9, lithium-10, and lithium11—all having half-lives of less than one second. Lithium was used (1932) as the target metal in the pioneering work of John Cockcroft and Ernest Walton in transmuting nuclei ...
... lithium-6 (7.5 percent); five radioactive isotopes have been prepared—lithium-5, lithium-8, lithium-9, lithium-10, and lithium11—all having half-lives of less than one second. Lithium was used (1932) as the target metal in the pioneering work of John Cockcroft and Ernest Walton in transmuting nuclei ...
Solutions_C19
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
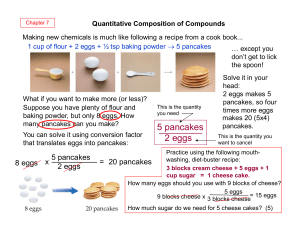
5 pancakes 2 eggs
... that is used up during the reaction, i.e. limiting reactant. One bicycle needs 1 frame, 1 seat and 2 wheels, therefore not more than 3 bicycles can be made. The number of seats is the limiting part (reactant); one frame and two wheels are parts in excess; 3 bicycles is the yield. ...
... that is used up during the reaction, i.e. limiting reactant. One bicycle needs 1 frame, 1 seat and 2 wheels, therefore not more than 3 bicycles can be made. The number of seats is the limiting part (reactant); one frame and two wheels are parts in excess; 3 bicycles is the yield. ...
Solutions_C19
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
STOICHIOMETRY via ChemLog - Small
... Amadeo Avogardro was not the first scientist who realized this number. However, he was the first scientist to sense the significance of the mole, so the number is named after him. Technically, a mole is an amount of substance that contains as many elementary entities as there are atoms in exactly 12 ...
... Amadeo Avogardro was not the first scientist who realized this number. However, he was the first scientist to sense the significance of the mole, so the number is named after him. Technically, a mole is an amount of substance that contains as many elementary entities as there are atoms in exactly 12 ...
Oxidation-Reduction Reactions
... Many elements simply combine with oxygen to form the oxide of that element. Heating magnesium in air allows it to combine with oxygen to form magnesium oxide. 2 Mg(s) + O2 (g) → 2MgO(s) Many compounds react with oxygen as well, often in very exothermic processes that are generally referred to as com ...
... Many elements simply combine with oxygen to form the oxide of that element. Heating magnesium in air allows it to combine with oxygen to form magnesium oxide. 2 Mg(s) + O2 (g) → 2MgO(s) Many compounds react with oxygen as well, often in very exothermic processes that are generally referred to as com ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.