CH. 3 - STOICHIOMETRY: CHEMICAL CALCULATIONS I. Molecular
... C2 x 1H2 x 2.500 = C2H5 III. Stoichiometric Equivalence and Reaction Stoichiometry A. stoichiometric coefficients - numbers placed in front of formulas to balance the equation, thereby indicating the combining ratios of reactants to products B. stoichiometric factors (mole ratios) - conversion facto ...
... C2 x 1H2 x 2.500 = C2H5 III. Stoichiometric Equivalence and Reaction Stoichiometry A. stoichiometric coefficients - numbers placed in front of formulas to balance the equation, thereby indicating the combining ratios of reactants to products B. stoichiometric factors (mole ratios) - conversion facto ...
CHEM_1305_Practice_Exam_2
... 4) Given that the only naturally occurring isotope of sodium is 23Na, what is its isotopic mass? A) 11.00 amu ...
... 4) Given that the only naturally occurring isotope of sodium is 23Na, what is its isotopic mass? A) 11.00 amu ...
Answers - Scioly.org
... 20. The student concludes that she has synthesized ethyl butanoate. Use evidence from the two experiments to support or to refute her claim. The peak of highest mass to charge ratio is approximately 116; therefore, the unknown molecule would have a molecular mass of 116. Ethyl butanoate has the chem ...
... 20. The student concludes that she has synthesized ethyl butanoate. Use evidence from the two experiments to support or to refute her claim. The peak of highest mass to charge ratio is approximately 116; therefore, the unknown molecule would have a molecular mass of 116. Ethyl butanoate has the chem ...
www.xtremepapers.net
... (CH3)3SiCl + C2H5O– → (CH3)3SiOC2H5 + Cl – which statements are likely to be true? ...
... (CH3)3SiCl + C2H5O– → (CH3)3SiOC2H5 + Cl – which statements are likely to be true? ...
Chemistry Syllabus
... If you are absent YOU ARE RESPONSIBLE to find out what you missed while you were out. You may use email to reach me our other classmates; You may also use your lab partner or other “class buddy”; You may also use the class website to find out what you missed while you were away. *****If your absen ...
... If you are absent YOU ARE RESPONSIBLE to find out what you missed while you were out. You may use email to reach me our other classmates; You may also use your lab partner or other “class buddy”; You may also use the class website to find out what you missed while you were away. *****If your absen ...
Exam 3 Review Key
... b) Lead’s primary mode of toxicity is its interference with enzyme function – it mimics other essential metals that take part in enzymatic reactions and displaces them. Considering the fact that sulfhydryl (-SH) groups are found on many enzymes, how might EDTA and DMSA work to treat lead poisoning? ...
... b) Lead’s primary mode of toxicity is its interference with enzyme function – it mimics other essential metals that take part in enzymatic reactions and displaces them. Considering the fact that sulfhydryl (-SH) groups are found on many enzymes, how might EDTA and DMSA work to treat lead poisoning? ...
Types of Chemical Reactions
... The ability of one metal to displace another depends on their relative ease of oxidation—a more active metal (one that is more easily oxidized) displaces a less active metal. In the first reaction above, lead is more active than copper. The relative activities of metals can be tabulated in an activi ...
... The ability of one metal to displace another depends on their relative ease of oxidation—a more active metal (one that is more easily oxidized) displaces a less active metal. In the first reaction above, lead is more active than copper. The relative activities of metals can be tabulated in an activi ...
CHEMISTRY 1710 - Practice Exam #2 (KATZ)
... _____24. How many moles of CO are contained in a 5.00 L tank at 155°C and 2.80 atm? A) 0.399 moles B) 1.10 moles C) 2.51 moles D) 0.455 moles _____25. What is the volume of 5.60 g of O2 at 7.78 atm and 415K? A) 1.53 L ...
... _____24. How many moles of CO are contained in a 5.00 L tank at 155°C and 2.80 atm? A) 0.399 moles B) 1.10 moles C) 2.51 moles D) 0.455 moles _____25. What is the volume of 5.60 g of O2 at 7.78 atm and 415K? A) 1.53 L ...
S8 + ___ F2 → ___ SF6 - Canvas by Instructure
... keep track of electrons in REDOX reactions. Oxidation numbers are SIMILAR to charge, but ...
... keep track of electrons in REDOX reactions. Oxidation numbers are SIMILAR to charge, but ...
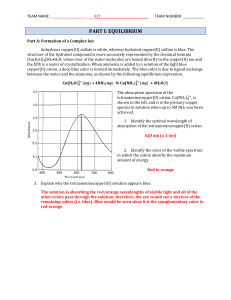
BONUS: Which line in the above graph represents G for the reaction
... which change will cause an increase in the pressure of CO2(g) when equilibrium is re-established? (A) ...
... which change will cause an increase in the pressure of CO2(g) when equilibrium is re-established? (A) ...
Chemistry EOC Review Name
... 117. What is an aqueous solution? 118. Distinguish between solvent and solute. 119. How are electrolytes different from nonelectrolytes? 120. Name three factors that increase the rate of solvation 121. What is meant by solubility? 122. What is the rule for determining if substances will soluble in e ...
... 117. What is an aqueous solution? 118. Distinguish between solvent and solute. 119. How are electrolytes different from nonelectrolytes? 120. Name three factors that increase the rate of solvation 121. What is meant by solubility? 122. What is the rule for determining if substances will soluble in e ...
PRE AP CHEMISTRY REVIEW PROBLEMS NON COLLEGE
... g. How many molecules are in a 1.20 mol sample of oxygen gas? How many oxygen atoms are in this sample? h. How many moles are in a 75.4 g sample of sulfur tetrachloride? i. What is the volume at STP of a 102 g sample of propane gas, C3H8? j. What is the mass of a sample that contains 1.22 × 1022 arg ...
... g. How many molecules are in a 1.20 mol sample of oxygen gas? How many oxygen atoms are in this sample? h. How many moles are in a 75.4 g sample of sulfur tetrachloride? i. What is the volume at STP of a 102 g sample of propane gas, C3H8? j. What is the mass of a sample that contains 1.22 × 1022 arg ...
A-level Paper 3 Practice Paper 3 - A
... (If you have been unable to answer part (d)(i) you may assume that three moles of manganate(VII) ions react with seven moles of iron(II) ethanedioate. This is not the ...
... (If you have been unable to answer part (d)(i) you may assume that three moles of manganate(VII) ions react with seven moles of iron(II) ethanedioate. This is not the ...
AP Chemistry
... a. when energy required to break bonds > energy released to form new bonds, +H (endothermic) 1. products at a higher energy state than reactants (weaker bonds) 2. surroundings lose energy (cool down) b. when energy required to break bonds < energy released to form new bonds, –H (exothermic) 1. pro ...
... a. when energy required to break bonds > energy released to form new bonds, +H (endothermic) 1. products at a higher energy state than reactants (weaker bonds) 2. surroundings lose energy (cool down) b. when energy required to break bonds < energy released to form new bonds, –H (exothermic) 1. pro ...
Enzymes - stephen fleenor
... like a key in a lock. • The part of the enzyme that fits with the substrate is called the active site. ...
... like a key in a lock. • The part of the enzyme that fits with the substrate is called the active site. ...
Spring Exam 2 - Chemistry
... Methane, CH4, reacts with excess oxygen to produce carbon dioxide, water, and heat. The standard molar enthalpy of combustion is –890.3 kJ. CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(l) What is the enthalpy change for the complete combustion of 32.1 g of methane? ...
... Methane, CH4, reacts with excess oxygen to produce carbon dioxide, water, and heat. The standard molar enthalpy of combustion is –890.3 kJ. CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(l) What is the enthalpy change for the complete combustion of 32.1 g of methane? ...
The student will
... experiments with partners, but a large percentage of their time outside the lab is spent in cooperative groups. These groups work together to solve advanced problems, complete projects, perform inquiry activities, and present information. The formal use of grouping has caused a significant increase ...
... experiments with partners, but a large percentage of their time outside the lab is spent in cooperative groups. These groups work together to solve advanced problems, complete projects, perform inquiry activities, and present information. The formal use of grouping has caused a significant increase ...
AP Review Chp 1 and Chp 2 Wed 10/9/2013 1. Near room
... I) Magnesium displaces copper from a dilute solution of copper ( II) sulfate; the pure copper will settle out of the solution. Mg(s) + CuSO4(aq) MgSO4(aq) + Cu(s) A copper(II) sulfate solution is mixed by dissolving 25.000 g of copper(II) sulfate, and then it is treated with an excess of magnesium ...
... I) Magnesium displaces copper from a dilute solution of copper ( II) sulfate; the pure copper will settle out of the solution. Mg(s) + CuSO4(aq) MgSO4(aq) + Cu(s) A copper(II) sulfate solution is mixed by dissolving 25.000 g of copper(II) sulfate, and then it is treated with an excess of magnesium ...
Unit 8 Note Packet
... 2. Balance a chemical equation based upon the law of conservation of matter. 3. Analyze or draw a graph for the energy change of a chemical reaction. 4. Calculate the heat of solution for a given compound. We are looking for: 1a. Given the word equation/sentence, translate it into a formula chemical ...
... 2. Balance a chemical equation based upon the law of conservation of matter. 3. Analyze or draw a graph for the energy change of a chemical reaction. 4. Calculate the heat of solution for a given compound. We are looking for: 1a. Given the word equation/sentence, translate it into a formula chemical ...
Chapter 3
... • The theoretical yield is the maximum amount of product that can be made. – In other words, it’s the amount of product possible as calculated through the stoichiometry problem. • This is different from the actual yield, which is the amount one actually produces and measures. ...
... • The theoretical yield is the maximum amount of product that can be made. – In other words, it’s the amount of product possible as calculated through the stoichiometry problem. • This is different from the actual yield, which is the amount one actually produces and measures. ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.