Formula and The Mole
... b) Hydrolysis is when a large molecule is broken down into two or more smaller molecules by reaction with water. c) Firstly heat the carbohydrate solutions with Benedict’s solution. If there is a colour change from blue to the formation of a red/orange precipitate, then either glucose, fructose or m ...
... b) Hydrolysis is when a large molecule is broken down into two or more smaller molecules by reaction with water. c) Firstly heat the carbohydrate solutions with Benedict’s solution. If there is a colour change from blue to the formation of a red/orange precipitate, then either glucose, fructose or m ...
Chemical Equations
... Decide, from the solubility table, which substances are soluble and which will form precipitates Expand the equation by dissociating all the soluble compounds into their free ions Check for any molecular substances such as acids and certain bases that react with water to produce ions (hydrolyse). Re ...
... Decide, from the solubility table, which substances are soluble and which will form precipitates Expand the equation by dissociating all the soluble compounds into their free ions Check for any molecular substances such as acids and certain bases that react with water to produce ions (hydrolyse). Re ...
Chemical Equations and Tests for anions
... atoms/molecules, but you may not change the formula by changing the small number after an atom • This method of balancing equations is known as balancing by inspection practice with question 6.1 (a) to (n) on p77 ...
... atoms/molecules, but you may not change the formula by changing the small number after an atom • This method of balancing equations is known as balancing by inspection practice with question 6.1 (a) to (n) on p77 ...
Definitions - Loreto Science
... • is a laboratory procedure where a a measured volume of one solution is added to a known volume of another solution until the reaction is complete. • (concentration of one solution known accurately at start) • (indicator used to show by colour change when reaction is complete) AG ...
... • is a laboratory procedure where a a measured volume of one solution is added to a known volume of another solution until the reaction is complete. • (concentration of one solution known accurately at start) • (indicator used to show by colour change when reaction is complete) AG ...
Name________________ Hour____ Chapter 11 Review 1. Name
... 2 atoms of solid aluminum react with 6 molecules of liquid water to produce 3 molecules of hydrogen gas and 2 formula units of solid aluminum hydroxide. 4. Write in symbols: 2 formula units of solid lead (IV) oxide decomposes in the presence of heat to produce 2 formula units of solid lead (II) oxid ...
... 2 atoms of solid aluminum react with 6 molecules of liquid water to produce 3 molecules of hydrogen gas and 2 formula units of solid aluminum hydroxide. 4. Write in symbols: 2 formula units of solid lead (IV) oxide decomposes in the presence of heat to produce 2 formula units of solid lead (II) oxid ...
2005 - NESACS
... 54. Which of the following statements about resonance structures are always true? Resonance can occur when I. A central atom of a molecule has a multiple bond on it. II. A central atom of a molecule has a lone pairs of electrons on it. III. The molecule's electronic geometry and molecular geometry a ...
... 54. Which of the following statements about resonance structures are always true? Resonance can occur when I. A central atom of a molecule has a multiple bond on it. II. A central atom of a molecule has a lone pairs of electrons on it. III. The molecule's electronic geometry and molecular geometry a ...
Analytical Chemistry
... Molarity = moles NaNO3 / volume of the solution in liters M = 0.1 mole / .500 liters = 0.200 Molar NaNO3 Since molarity involves a basis of solution volume, it is apparent that the molarity of a solution will change as volume changes which is associated with changes in temperature. Formal Concentrat ...
... Molarity = moles NaNO3 / volume of the solution in liters M = 0.1 mole / .500 liters = 0.200 Molar NaNO3 Since molarity involves a basis of solution volume, it is apparent that the molarity of a solution will change as volume changes which is associated with changes in temperature. Formal Concentrat ...
Chemistry II Exams and Answer Keys 2015 Season
... If 2.0 mol of A are converted into products at a pressure of 1.25 atm and 1000.0°C, calculate the ΔE for the reaction? 1 liter × atm = 101.3 J A. 220 kJ B. −220 kJ C. 6.20 kJ D. −6.20 kJ 19. When elements with electron configuration 1s2 2s2 2p6 3s2 3p2 and 1s2 2s2 2p4 combine, they form a(n) _______ ...
... If 2.0 mol of A are converted into products at a pressure of 1.25 atm and 1000.0°C, calculate the ΔE for the reaction? 1 liter × atm = 101.3 J A. 220 kJ B. −220 kJ C. 6.20 kJ D. −6.20 kJ 19. When elements with electron configuration 1s2 2s2 2p6 3s2 3p2 and 1s2 2s2 2p4 combine, they form a(n) _______ ...
1. Review (MC problems, due Monday) 2. - mvhs
... 20. Account for each of the following in terms of principles of atomic structure, including the number, properties, and arrangements of subatomic particles. (a) The second ionization energy of sodium is about three times greater than the second ionization energy of magnesium. (b) The difference betw ...
... 20. Account for each of the following in terms of principles of atomic structure, including the number, properties, and arrangements of subatomic particles. (a) The second ionization energy of sodium is about three times greater than the second ionization energy of magnesium. (b) The difference betw ...
Chapters 7/8 Worksheet 1
... 1. Using electronegativity values, determine the type of each compound as being ionic, polar covalent, or nonpolar covalent. CH4 ...
... 1. Using electronegativity values, determine the type of each compound as being ionic, polar covalent, or nonpolar covalent. CH4 ...
Gas Laws
... 18. The rate of effusion of an unknown gas was determined to be 2.92 times faster than that of ammonia. What is the approximate molecular weight of the unknown gas? 19. In the reaction, N2 + H2 NH3, how many mL of nitrogen, measured at STP, are required to produce 400 mL of NH3, measured at STP? H ...
... 18. The rate of effusion of an unknown gas was determined to be 2.92 times faster than that of ammonia. What is the approximate molecular weight of the unknown gas? 19. In the reaction, N2 + H2 NH3, how many mL of nitrogen, measured at STP, are required to produce 400 mL of NH3, measured at STP? H ...
Preparation and Characterization of a Dip-Coated SnO2
... A new method for the synthesis of SnO2 is proposed and thin films are prepared by a dip-coating method. In the present paper we report that these SnO2 films exhibit a reversible electrochemical insertion of lithium ions while maintaining high optical transmissivity. These films can be used as transp ...
... A new method for the synthesis of SnO2 is proposed and thin films are prepared by a dip-coating method. In the present paper we report that these SnO2 films exhibit a reversible electrochemical insertion of lithium ions while maintaining high optical transmissivity. These films can be used as transp ...
Chemistr.e1a.chapter.4.new2015
... • The following reaction that you have seen before in class and the laboratory is neither a precipitation reaction nor an acid-base reaction. Cu (s) + ½ O2 (g) " CuO (s) The reaction above is one where electrons are transferred from one element to another during the reaction. This kind of reaction i ...
... • The following reaction that you have seen before in class and the laboratory is neither a precipitation reaction nor an acid-base reaction. Cu (s) + ½ O2 (g) " CuO (s) The reaction above is one where electrons are transferred from one element to another during the reaction. This kind of reaction i ...
Gen Chem Final--review problems Fall 2006
... In a solution calorimeter, 50.0 mL of 0.100 M AgNO3 solution and 50.0 mL of 0.100 M HCl are mixed. The following reaction occurs: Ag+(aq) + Cl-(aq) Î AgCl(s) If the two solutions were initially at 22.6°C and the final temperature is 23.4°C, calculate qrxn and ΔHrxn. Assume that the surroundings are ...
... In a solution calorimeter, 50.0 mL of 0.100 M AgNO3 solution and 50.0 mL of 0.100 M HCl are mixed. The following reaction occurs: Ag+(aq) + Cl-(aq) Î AgCl(s) If the two solutions were initially at 22.6°C and the final temperature is 23.4°C, calculate qrxn and ΔHrxn. Assume that the surroundings are ...
Dr David`s Chemistry Revision Themes
... A 100 cm3 of tetrachloromethane is added to the above mixture. After shaking and allowing to settle, two liquid layers form. Describe and explain the result. Tetrachloromethane is immiscible with water and forms a bottom layer since it is denser than water. It also preferentially dissolves the iodin ...
... A 100 cm3 of tetrachloromethane is added to the above mixture. After shaking and allowing to settle, two liquid layers form. Describe and explain the result. Tetrachloromethane is immiscible with water and forms a bottom layer since it is denser than water. It also preferentially dissolves the iodin ...
Science 9
... In her notebook, the student recorded the final mass of the products, it was 140 g. Did this reaction conserve mass? Explain your answer. ...
... In her notebook, the student recorded the final mass of the products, it was 140 g. Did this reaction conserve mass? Explain your answer. ...
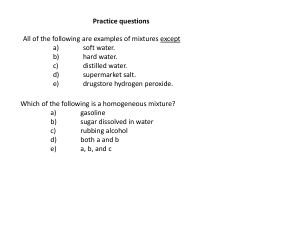
Practice questions
... a) neutrons. b) atomic number. c) nuclear charge. d) electron configuration. e) number of protons. ...
... a) neutrons. b) atomic number. c) nuclear charge. d) electron configuration. e) number of protons. ...
Molarity Practice Worksheet
... Explain how to make at least one liter of a 1.25 molar ammonium hydroxide solution. ...
... Explain how to make at least one liter of a 1.25 molar ammonium hydroxide solution. ...
File
... eg. butane reacts with oxygen to produce carbon dioxide and water 4. Chemical Change: a chemical reaction; a change in which at least one or more new substances (products) are formed. The products have different properties from the starting ...
... eg. butane reacts with oxygen to produce carbon dioxide and water 4. Chemical Change: a chemical reaction; a change in which at least one or more new substances (products) are formed. The products have different properties from the starting ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.