Carbohydrates - Midlands State University
... 5. Calculate the expected sucrose concentration from 3 and 4 above and graph the boiling point vs the expected and observed sucrose concentration on the same graph as described for salt in 2c. 6.a) Prepare 50g of 10% (W/W) solution of sucrose and a 10% of NaCl . b) Determine the freezing point of th ...
... 5. Calculate the expected sucrose concentration from 3 and 4 above and graph the boiling point vs the expected and observed sucrose concentration on the same graph as described for salt in 2c. 6.a) Prepare 50g of 10% (W/W) solution of sucrose and a 10% of NaCl . b) Determine the freezing point of th ...
CHM 50 Exam 1 Review Name Due Tuesday 9/29/09 Exam 1 will
... a. Sodium metal plus water yields hydrogen gas and an aqueous sodium hydroxide solution. b. Potassium chlorate when heated yields potassium chlorate plus oxygen gas. c. An aqueous phosphoric acid solution plus an aqueous calcium hydroxide solution yields water and solid calcium phosphate. 5. A carta ...
... a. Sodium metal plus water yields hydrogen gas and an aqueous sodium hydroxide solution. b. Potassium chlorate when heated yields potassium chlorate plus oxygen gas. c. An aqueous phosphoric acid solution plus an aqueous calcium hydroxide solution yields water and solid calcium phosphate. 5. A carta ...
Differentiated Chemistry First Term Test Review
... When glucose (C6H12O6) reacts with oxygen gas in the body, carbon dioxide, water, and energy are produced. How many grams of carbon dioxide would be produced if 45 grams of glucose completely reacted with the oxygen gas? (A) 1.5 g (B) 1.8 g (C) 11 g (D) 66 g (E) 12000 g ...
... When glucose (C6H12O6) reacts with oxygen gas in the body, carbon dioxide, water, and energy are produced. How many grams of carbon dioxide would be produced if 45 grams of glucose completely reacted with the oxygen gas? (A) 1.5 g (B) 1.8 g (C) 11 g (D) 66 g (E) 12000 g ...
Chapter 3
... 62. Allicin is the compound responsible for the characteristic smell of garlic. An analysis of the compound gives the following percent composition by mass: C: 44.4 percent; H: 6.21 percent; S: 39.5 percent; O: 9.86 percent. What is its molecular formula given that its molar mass is about 162 g? A) ...
... 62. Allicin is the compound responsible for the characteristic smell of garlic. An analysis of the compound gives the following percent composition by mass: C: 44.4 percent; H: 6.21 percent; S: 39.5 percent; O: 9.86 percent. What is its molecular formula given that its molar mass is about 162 g? A) ...
this page as a PDF
... to “trap” or seal hydrogen in the base material. Once the hydrogen is sealed in the fastener, it is more likely to produce an embrittlement failure. Mechanical coatings are more porous (less dense). Therefore, any hydrogen in the base material of a mechanically coated fastener will have a better opp ...
... to “trap” or seal hydrogen in the base material. Once the hydrogen is sealed in the fastener, it is more likely to produce an embrittlement failure. Mechanical coatings are more porous (less dense). Therefore, any hydrogen in the base material of a mechanically coated fastener will have a better opp ...
syllabus details - hrsbstaff.ednet.ns.ca
... Many chemical reactions are reversible and never go to completion. Equilibrium can be approached from both directions. For a system in equilibrium the rate of the forward reaction equals the rate of the reverse reaction and the concentration of all reactants and products remain constant. The system ...
... Many chemical reactions are reversible and never go to completion. Equilibrium can be approached from both directions. For a system in equilibrium the rate of the forward reaction equals the rate of the reverse reaction and the concentration of all reactants and products remain constant. The system ...
The LONESTARTM Portable Analyzer
... Figure 16 DMMP Monomer and dimer formation at different concentrations The likelihood of ionization is governed by the analyte’s affinity towards protons and electrons (Table 2 and Table 3 respectively). In complex mixtures where more than one chemical is present, competition for the available charg ...
... Figure 16 DMMP Monomer and dimer formation at different concentrations The likelihood of ionization is governed by the analyte’s affinity towards protons and electrons (Table 2 and Table 3 respectively). In complex mixtures where more than one chemical is present, competition for the available charg ...
Practice Problem Set #6
... 1. Write balanced chemical equations for the reaction of hydrogen gas with oxygen, chlorine, and nitrogen. 2. Write a balanced chemical equation for the preparation of H2 (and CO) by the reaction of CH4 and water. Using a table of thermodynamic data, calculate ∆H°, ∆G°, and ∆S° for this reaction. ...
... 1. Write balanced chemical equations for the reaction of hydrogen gas with oxygen, chlorine, and nitrogen. 2. Write a balanced chemical equation for the preparation of H2 (and CO) by the reaction of CH4 and water. Using a table of thermodynamic data, calculate ∆H°, ∆G°, and ∆S° for this reaction. ...
TDB-5: Standards and conventions for TDB publications
... ν+ Xν− which dissociates into ν ± (= ν+ +ν− ) ions, in an aqueous solution with concentration m, the individual cationic molality and activity coefficient are m + (= ν+ m) and γ+ (= a+ /m + ). A similar definition is used for the anionic symbols. Electrical neutrality requires that ν + z + = ν− z − ...
... ν+ Xν− which dissociates into ν ± (= ν+ +ν− ) ions, in an aqueous solution with concentration m, the individual cationic molality and activity coefficient are m + (= ν+ m) and γ+ (= a+ /m + ). A similar definition is used for the anionic symbols. Electrical neutrality requires that ν + z + = ν− z − ...
Chemistry exam review
... 2. A student mixes two chemicals in a test tube. The test tube turns hot and bubbles appear. What indicators of chemical reaction is the student observing? a. Change in color and formation of precipitate. b. Change in color and formation of gas. c. Change in temperature and formation of precipitate. ...
... 2. A student mixes two chemicals in a test tube. The test tube turns hot and bubbles appear. What indicators of chemical reaction is the student observing? a. Change in color and formation of precipitate. b. Change in color and formation of gas. c. Change in temperature and formation of precipitate. ...
3C95 Chemistry 12 2015-2016 (Lockwood)
... RATIONALE: Chemistry is the science, which deals with the properties and reactions of materials; it is concerned with the identification, characterization and transformation of matter and with the energy changes accompanying these transformations. Chemical science focuses on the structure and intera ...
... RATIONALE: Chemistry is the science, which deals with the properties and reactions of materials; it is concerned with the identification, characterization and transformation of matter and with the energy changes accompanying these transformations. Chemical science focuses on the structure and intera ...
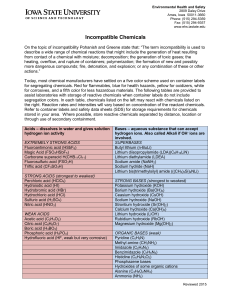
Incompatible Chemicals
... Today, most chemical manufacturers have settled on a five color scheme used on container labels for segregating chemicals. Red for flammables, blue for health hazards, yellow for oxidizers, white for corrosives, and a fifth color for less hazardous materials. The following tables are provided to ass ...
... Today, most chemical manufacturers have settled on a five color scheme used on container labels for segregating chemicals. Red for flammables, blue for health hazards, yellow for oxidizers, white for corrosives, and a fifth color for less hazardous materials. The following tables are provided to ass ...
Stoichiometry Notes
... an unknown substance and the solute of the standard solution. The completion of the reaction is indicated by the end point of the reaction, which is observed by the colour change either due to the indicator or due to the solute itself. Whether the reactions during the analysis are either between an ...
... an unknown substance and the solute of the standard solution. The completion of the reaction is indicated by the end point of the reaction, which is observed by the colour change either due to the indicator or due to the solute itself. Whether the reactions during the analysis are either between an ...
Chemistry 534
... What temperature change will be experienced by a 250.0 mL mixture (final volume) if it resulted from the neutralization of equal amounts of NaOH and HI? Molar heat of neutralization of NaOH= -200.0 kJ/mole Concentration of NaOH = 0.30 g/L ...
... What temperature change will be experienced by a 250.0 mL mixture (final volume) if it resulted from the neutralization of equal amounts of NaOH and HI? Molar heat of neutralization of NaOH= -200.0 kJ/mole Concentration of NaOH = 0.30 g/L ...
Test Booklet
... 10 According to this balanced chemical equation, what volume of C2 H2 is required to form 40.0 L of CO2 ? 2C2 H2 (g) + 5O2 (g) → 2H2 O (g) + 4CO2 (g) ...
... 10 According to this balanced chemical equation, what volume of C2 H2 is required to form 40.0 L of CO2 ? 2C2 H2 (g) + 5O2 (g) → 2H2 O (g) + 4CO2 (g) ...
CHEMISTRY SEMESTER ONE LAB 1 Lab 1: Stoichiometry and
... 7. Decant the wash water from the copper and add 10 more mL of distilled water, swirl and decant again. Put the liquid from these two washes in the labeled waste container provided in your kit. 8. Now wash the copper with several mL of acetone (Be careful! Acetone is very flammable). Swirl and allow ...
... 7. Decant the wash water from the copper and add 10 more mL of distilled water, swirl and decant again. Put the liquid from these two washes in the labeled waste container provided in your kit. 8. Now wash the copper with several mL of acetone (Be careful! Acetone is very flammable). Swirl and allow ...
Chemistry 432: Final Exam Review Sheet
... 7. Oxidation-reduction reactions and oxidation numbers: (8 questions) Calculating oxidation numbers, recognizing substances which are being oxidized and those being reduced, recognizing redox reactions. Balancing Redox reactions. Electrochemistry. Handout. 8. Physical and Chemical Equilibrium: Writi ...
... 7. Oxidation-reduction reactions and oxidation numbers: (8 questions) Calculating oxidation numbers, recognizing substances which are being oxidized and those being reduced, recognizing redox reactions. Balancing Redox reactions. Electrochemistry. Handout. 8. Physical and Chemical Equilibrium: Writi ...
Final Exam Practice Questions for General Chemistry NOTICE TO
... a) precipitation, redox, and acid-base reactions, respectively. b) unbalanced reactions. c) precipitation, acid-base, and redox reactions, respectively. d) redox, acid-base, and precipitation reactions, respectively. e) acid-base reactions. 2. If you were to make an aqueous solution of silver nitrat ...
... a) precipitation, redox, and acid-base reactions, respectively. b) unbalanced reactions. c) precipitation, acid-base, and redox reactions, respectively. d) redox, acid-base, and precipitation reactions, respectively. e) acid-base reactions. 2. If you were to make an aqueous solution of silver nitrat ...
Tutorial 1
... 10. Urea [(NH2)2CO] is prepares by reacting ammonia with carbon dioxide: NH3 + CO2 (NH2)2CO + H2O (not balanced) In one process 637.2 g of NH3 are allowed to react with 1142 g of CO2. a. Which of the reactants is limiting reagent? b. Calculate, the mass of (NH2)2CO formed? And excess reagent (in g ...
... 10. Urea [(NH2)2CO] is prepares by reacting ammonia with carbon dioxide: NH3 + CO2 (NH2)2CO + H2O (not balanced) In one process 637.2 g of NH3 are allowed to react with 1142 g of CO2. a. Which of the reactants is limiting reagent? b. Calculate, the mass of (NH2)2CO formed? And excess reagent (in g ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.