9 free IB Chem labs (sent to OCC) - VicPark-IBRoundtable-2009
... the tubing inside the cylinder. 2 people should be operating the apparatus and 1 person should be recording data. 7. Take volume measurements every 30 seconds until the reaction finishes. 8. The final volume of gas is not only hydrogen gas, it has water vapour as well, because the gas was bubbled th ...
... the tubing inside the cylinder. 2 people should be operating the apparatus and 1 person should be recording data. 7. Take volume measurements every 30 seconds until the reaction finishes. 8. The final volume of gas is not only hydrogen gas, it has water vapour as well, because the gas was bubbled th ...
Praktikum in Allgemeiner Chemie für Biologen und Pharmazeuten
... soot producing flame is obtained. It has the lowest temperature but should not be used for gentle heating since it would spoil the equipment with soot. This air supply position, however, is most suitable for the ignition. When the air supply is opened slightly the yellow emission disappears and the ...
... soot producing flame is obtained. It has the lowest temperature but should not be used for gentle heating since it would spoil the equipment with soot. This air supply position, however, is most suitable for the ignition. When the air supply is opened slightly the yellow emission disappears and the ...
Chemistry B2A Chapter 18 Oxidation
... In some reactions, it is not easy to see the electron loss and gain, so chemists developed another definition of oxidation and reduction: Oxidation is the gain of oxygen atoms and/or the loss of hydrogen atoms. Reduction is the loss of oxygen atoms and/or the gain of hydrogen atoms. CH4(g) + 2O2(g) ...
... In some reactions, it is not easy to see the electron loss and gain, so chemists developed another definition of oxidation and reduction: Oxidation is the gain of oxygen atoms and/or the loss of hydrogen atoms. Reduction is the loss of oxygen atoms and/or the gain of hydrogen atoms. CH4(g) + 2O2(g) ...
4. bonding - New Hartford Central Schools
... 3.Place the polyatomic ion in parentheses Crisscross the oxidation numbers and omit the charge signs. Write the numbers below the symbols as subscripts. Al2(SO4)3 The sum of the oxidation numbers of all of the atoms in a compound is always zero. 4. When each element has the same oxidation number, th ...
... 3.Place the polyatomic ion in parentheses Crisscross the oxidation numbers and omit the charge signs. Write the numbers below the symbols as subscripts. Al2(SO4)3 The sum of the oxidation numbers of all of the atoms in a compound is always zero. 4. When each element has the same oxidation number, th ...
stoichiometry - einstein classes
... It is based on law of equivalence which is explained as follows : Law of chemical equivalents : In a chemical reaction the equivalents of all the species (reactants or products) are equal to each other provided none of these compounds is in excess. N1V1 = N2V2 (when normalities and volumes are given ...
... It is based on law of equivalence which is explained as follows : Law of chemical equivalents : In a chemical reaction the equivalents of all the species (reactants or products) are equal to each other provided none of these compounds is in excess. N1V1 = N2V2 (when normalities and volumes are given ...
Answers to Selected Questions and Problems
... (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i) subatomic particle; (j) alkali metal; (k) periodic table Dalton used the laws of conservation of mass (Lavoisier) and definite proportions (Proust). They differ in their atomic masses and chemical propert ...
... (d) main-group element; (e) relative atomic mass; (f) mass number; (g) isotope; (h) cation; (i) subatomic particle; (j) alkali metal; (k) periodic table Dalton used the laws of conservation of mass (Lavoisier) and definite proportions (Proust). They differ in their atomic masses and chemical propert ...
Full research publication
... 6.5h10-5M. As a reference standard of antiradical activity of Trolox was used (production Sigma-Aldrich) (Table 1). Table 1 Antiradical activity of methyl (2Z) - [(3Z) -3- (2- oksopentiliden) -3,4-digidrohinoksalin2 (1H) - ylidene] etanoat (2) ...
... 6.5h10-5M. As a reference standard of antiradical activity of Trolox was used (production Sigma-Aldrich) (Table 1). Table 1 Antiradical activity of methyl (2Z) - [(3Z) -3- (2- oksopentiliden) -3,4-digidrohinoksalin2 (1H) - ylidene] etanoat (2) ...
Word - chemmybear.com
... At 600K, the equilibrium constant Kp is 0.060. In a vessel at 600K, there is a mixture of all three gases. The partial pressure of NOCl is 675 torr, the partial pressure of NO is 43 torr and the partial pressure of chlorine is 23 torr. a. What is the value of the reaction quotient? ...
... At 600K, the equilibrium constant Kp is 0.060. In a vessel at 600K, there is a mixture of all three gases. The partial pressure of NOCl is 675 torr, the partial pressure of NO is 43 torr and the partial pressure of chlorine is 23 torr. a. What is the value of the reaction quotient? ...
Honors Chemistry - Stout Middle School
... h. Formula unit i. Monatomic ion j. Oxidation number (charge) k. Polyatomic ion l. Electron sea model m. Octet n. Crystal lattice o. Metallic bond p. Lattice energy q. Alloy 2. Know how cations and anions are formed. 3. Know what elements form cations and anions. 4. Be able to predict oxidation numb ...
... h. Formula unit i. Monatomic ion j. Oxidation number (charge) k. Polyatomic ion l. Electron sea model m. Octet n. Crystal lattice o. Metallic bond p. Lattice energy q. Alloy 2. Know how cations and anions are formed. 3. Know what elements form cations and anions. 4. Be able to predict oxidation numb ...
South Pasadena · AP Chemistry
... At 600K, the equilibrium constant Kp is 0.060. In a vessel at 600K, there is a mixture of all three gases. The partial pressure of NOCl is 675 torr, the partial pressure of NO is 43 torr and the partial pressure of chlorine is 23 torr. a. What is the value of the reaction quotient? ...
... At 600K, the equilibrium constant Kp is 0.060. In a vessel at 600K, there is a mixture of all three gases. The partial pressure of NOCl is 675 torr, the partial pressure of NO is 43 torr and the partial pressure of chlorine is 23 torr. a. What is the value of the reaction quotient? ...
Understanding the Role of Aqueous Solution in Chemical Reactions
... studies of two prototype aqueous chemical reactions. These reactions are not only of fundamental interest, but have also a significant importance in technological applications. In our study we employed molecular simulation of an accurate atomistic model, yielding a detailed picture of the structure ...
... studies of two prototype aqueous chemical reactions. These reactions are not only of fundamental interest, but have also a significant importance in technological applications. In our study we employed molecular simulation of an accurate atomistic model, yielding a detailed picture of the structure ...
Chapter 1, 2, 3, 4 Percent Composition, Ions, Stoichiometry
... equation above. If 0.10 mole of powdered silver is added to 10 ml of 6.0-molar nitric acid, the number of moles of NO gas that can be formed is: 3Ag(s) + 4HNO3 3AgNO3 + NO(g) + 2H2O (A) 0.015 mole (B) 0.020 mole (C) 0.030 mole (D) 0.045 mole (E) 0.090 mole ...
... equation above. If 0.10 mole of powdered silver is added to 10 ml of 6.0-molar nitric acid, the number of moles of NO gas that can be formed is: 3Ag(s) + 4HNO3 3AgNO3 + NO(g) + 2H2O (A) 0.015 mole (B) 0.020 mole (C) 0.030 mole (D) 0.045 mole (E) 0.090 mole ...
Calculating molar volume
... Step 3: Using the number of moles in step 1, choose one reactant and work out the number of moles of the other reactant needed to react with it: ...
... Step 3: Using the number of moles in step 1, choose one reactant and work out the number of moles of the other reactant needed to react with it: ...
Document
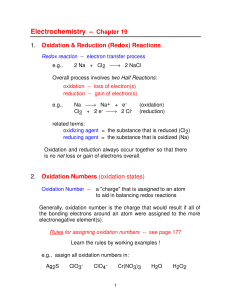
... 8. Multiply balanced half-reactions by appropriate coefficients so that the number of electrons are equal. 9. Add the half-reactions together. 10. Cancel species that appear on both sides to get the balanced Net ...
... 8. Multiply balanced half-reactions by appropriate coefficients so that the number of electrons are equal. 9. Add the half-reactions together. 10. Cancel species that appear on both sides to get the balanced Net ...
In Situ Vanadium K-Edge and Tungsten LIII-Edge X
... X Abstract published in AdVance ACS Abstracts, November 1, 1996. ...
... X Abstract published in AdVance ACS Abstracts, November 1, 1996. ...
- Deans Community High School
... c) Gold and platinum both catalyse the reaction. For the forward reaction E A using gold is 30 kJ, while EA using platinum is 40 kJ. i) using different dotted lines add this information to the graph. ii) which is the better catalyst for the reaction? Explain your choice. d) The gold and platinum cat ...
... c) Gold and platinum both catalyse the reaction. For the forward reaction E A using gold is 30 kJ, while EA using platinum is 40 kJ. i) using different dotted lines add this information to the graph. ii) which is the better catalyst for the reaction? Explain your choice. d) The gold and platinum cat ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.