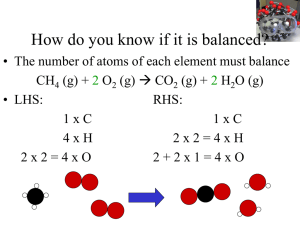
1 Chemical Reactions and Equations
... (ii) Addition of colourless lead nitrate solution to potassium iodide solution gives yellow coloured precipitate (lead iodide). So there is a change of colour. (iii) Addition of dilute hydrochloric acid to zinc granules gives a gas (H2) with effervescence. Q. 4. How do you show that respiration is a ...
... (ii) Addition of colourless lead nitrate solution to potassium iodide solution gives yellow coloured precipitate (lead iodide). So there is a change of colour. (iii) Addition of dilute hydrochloric acid to zinc granules gives a gas (H2) with effervescence. Q. 4. How do you show that respiration is a ...
INDIAN JOURNAL OF CHEMISTRY
... The stoichiometry of the title reaction is expressed by the Equation, MnO 4 + 2C6H5HP(O)OH + 4H+ Mn(III) + 2C6H5PO(OH)2 + 2H2O. The kinetics and the rapid scan of the reaction mixture suggest the formation of the complexes by C6H5HP(O)OH(C1, K1) and C6H5HP(O)O (C2, K2) with MnO4 ion. The equilib ...
... The stoichiometry of the title reaction is expressed by the Equation, MnO 4 + 2C6H5HP(O)OH + 4H+ Mn(III) + 2C6H5PO(OH)2 + 2H2O. The kinetics and the rapid scan of the reaction mixture suggest the formation of the complexes by C6H5HP(O)OH(C1, K1) and C6H5HP(O)O (C2, K2) with MnO4 ion. The equilib ...
Redox Reactions and Electrochemistry
... Standard Electrode Potentials In electronics and electricity theory, a voltage is a measurement of the potential to do electrical work measured between two points in a circuit. Absolute measurements of potential (voltage) at a single point are meaningless, UNLESS, they are measured against some know ...
... Standard Electrode Potentials In electronics and electricity theory, a voltage is a measurement of the potential to do electrical work measured between two points in a circuit. Absolute measurements of potential (voltage) at a single point are meaningless, UNLESS, they are measured against some know ...
C - mvhs-fuhsd.org
... D. Atoms have a positively charged nucleus surrounded by an electron cloud. E. No two electrons in one atom can have the same four quantum numbers. 65. The following properties are observed for an unknown element, Z: at room temperature, it is gray, lustrous solid. The compound ZCl2 dissolves in wat ...
... D. Atoms have a positively charged nucleus surrounded by an electron cloud. E. No two electrons in one atom can have the same four quantum numbers. 65. The following properties are observed for an unknown element, Z: at room temperature, it is gray, lustrous solid. The compound ZCl2 dissolves in wat ...
MEDICAL CHEMISTRY STUDY GUIDE
... of biological membranes. The values that characterize the quantitative composition of solutions. Preparation of solutions of a given quantitative composition. 3.2. Overview Solution is a homogeneous mixture of two or more substances, consisting of ions or molecules. This term is usually used to desc ...
... of biological membranes. The values that characterize the quantitative composition of solutions. Preparation of solutions of a given quantitative composition. 3.2. Overview Solution is a homogeneous mixture of two or more substances, consisting of ions or molecules. This term is usually used to desc ...
Chapter 15 - Cengage Learning
... ions. To understand why a crystal of potassium sulfate breaks apart into ions, we need to consider the nature of the water molecule. Water molecules are polar; that is, one end of the molecule has a partial positive charge, and the other end has a partial negative charge. The positive potassium ion ...
... ions. To understand why a crystal of potassium sulfate breaks apart into ions, we need to consider the nature of the water molecule. Water molecules are polar; that is, one end of the molecule has a partial positive charge, and the other end has a partial negative charge. The positive potassium ion ...
H2-rich fluids from serpentinization: Geochemical and biotic
... rapidly dissolve in ground waters, making the water supersaturated in serpentine and brucite that precipitate slowly (40). We cannot simply apply reaction 3 because kinetically controlled supersaturated reactants occur on both sides. It has not escaped us that disequilibrium between dissolved Fe(II) ...
... rapidly dissolve in ground waters, making the water supersaturated in serpentine and brucite that precipitate slowly (40). We cannot simply apply reaction 3 because kinetically controlled supersaturated reactants occur on both sides. It has not escaped us that disequilibrium between dissolved Fe(II) ...
Briefing Session on 2012 HKDSE Examination (December 2012)
... Explain qualitatively the effect on the initial rate of the reaction of replacing the 2.0 M HCl(aq) with 2.0 M H2SO4(aq). HCl is a monobasic acid, while H2SO4 is a dibasic acid. Initial rate increases as the concentration of H+ increases in 2.0 M H2SO4. ...
... Explain qualitatively the effect on the initial rate of the reaction of replacing the 2.0 M HCl(aq) with 2.0 M H2SO4(aq). HCl is a monobasic acid, while H2SO4 is a dibasic acid. Initial rate increases as the concentration of H+ increases in 2.0 M H2SO4. ...
answers to part a of the national high school
... This question is concerned with the categories of substances1 shown below. Note that the letters here refer to the answer keys and not to the official reference codes for the different classes of compounds. A. Flammable and combustible material2 (Class B), e.g. white phosphorus, acetone and butane. ...
... This question is concerned with the categories of substances1 shown below. Note that the letters here refer to the answer keys and not to the official reference codes for the different classes of compounds. A. Flammable and combustible material2 (Class B), e.g. white phosphorus, acetone and butane. ...
CHEM110P1_06_2015_Y_P1
... A student used a titration to determine whether an unknown sample was malonic acid (CH2(COOH)2, molar mass = 104.1 g mol–1). The student weighed 1.08 g of the unknown acid and transferred it to a 250.0 mL volumetric flask and prepared a standard solution. The burette was filled with 0.09970 M NaOH s ...
... A student used a titration to determine whether an unknown sample was malonic acid (CH2(COOH)2, molar mass = 104.1 g mol–1). The student weighed 1.08 g of the unknown acid and transferred it to a 250.0 mL volumetric flask and prepared a standard solution. The burette was filled with 0.09970 M NaOH s ...
Mole calculations File
... • volume 12 g = mass/density … unit …convert to m3 • volume occupied by an atom …assumption cubic or spherical? • atoms in 12 g = vol 12g/vol atom • Approx 1x1024…should be 6.02x1023 mol-1 ...
... • volume 12 g = mass/density … unit …convert to m3 • volume occupied by an atom …assumption cubic or spherical? • atoms in 12 g = vol 12g/vol atom • Approx 1x1024…should be 6.02x1023 mol-1 ...
1 • Introduction The Scientific Method (1 of 20) 1
... • metals often are written with the ° symbol to emphasize that the metal is in the neutral elemental state, not an ion. • some compounds have common names that you should just know... water, H 2 O; ammonia, NH3 ; methane, CH4 • remember the seven diatomic elements so they can be written as diatomic ...
... • metals often are written with the ° symbol to emphasize that the metal is in the neutral elemental state, not an ion. • some compounds have common names that you should just know... water, H 2 O; ammonia, NH3 ; methane, CH4 • remember the seven diatomic elements so they can be written as diatomic ...
SEKOLAH MENENGAH KEBANGSAAN RAJA PEREMPUAN, IPOH
... a. the similarities in chemical properties of lithium, sodium and potassium b. the relationship between the chemical properties of Group 1 elements and their electron arrangements Study the reaction of lithium, sodium and potassium with chlorine and bromine through computer simulation. Discuss chang ...
... a. the similarities in chemical properties of lithium, sodium and potassium b. the relationship between the chemical properties of Group 1 elements and their electron arrangements Study the reaction of lithium, sodium and potassium with chlorine and bromine through computer simulation. Discuss chang ...
WJEC CBAC AS/A LEVEL GCE in Chemistry REVISION AID UNIT 1
... murder of the Russian, Alexander Litvinenko, in 2006, was brought about by the alpha emitter polonium-210. Exposure to all three radiations can cause irreversible organ damage. ...
... murder of the Russian, Alexander Litvinenko, in 2006, was brought about by the alpha emitter polonium-210. Exposure to all three radiations can cause irreversible organ damage. ...
Summaries of Review Topics for AP Chemistry
... The charge (or oxidation number/state) of a monatomic ion is equal to the number of electrons that were transferred from an atom. Ex: Ca2+ (the Calcium atom lost 2 valence electrons) and S2- (S gained 2 electrons.) (3) An ion that contains more than one atom is called a polyatomic ion. The charge of ...
... The charge (or oxidation number/state) of a monatomic ion is equal to the number of electrons that were transferred from an atom. Ex: Ca2+ (the Calcium atom lost 2 valence electrons) and S2- (S gained 2 electrons.) (3) An ion that contains more than one atom is called a polyatomic ion. The charge of ...
Chem expo 12
... across the unit: a summary report including annotations of three related practical activities worth 50% of the marks available, a written report on one practical activity from the other Area of Study (25%) and another task such as the analysis of data using structured questions (25%). ...
... across the unit: a summary report including annotations of three related practical activities worth 50% of the marks available, a written report on one practical activity from the other Area of Study (25%) and another task such as the analysis of data using structured questions (25%). ...
Marking Scheme - State Examination Commission
... This process is overseen by the Chief Examiner, usually assisted by a Chief Advising Examiner. The Chief Examiner is the final authority regarding whether or not the marking scheme has been correctly applied to any piece of candidate work. Marking schemes are working documents. While a draft marking ...
... This process is overseen by the Chief Examiner, usually assisted by a Chief Advising Examiner. The Chief Examiner is the final authority regarding whether or not the marking scheme has been correctly applied to any piece of candidate work. Marking schemes are working documents. While a draft marking ...
9791/02 UNIVERSITY OF CAMBRIDGE INTERNATIONAL
... What are the two most important safety measures to guard against the hazards of these materials? You can assume that the experimenters are wearing gloves, eye protection and lab coats. ...
... What are the two most important safety measures to guard against the hazards of these materials? You can assume that the experimenters are wearing gloves, eye protection and lab coats. ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.