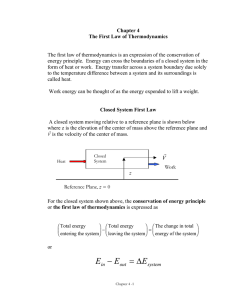
Chapter 4: The First Law of Thermodynamics
... Conservation of Energy for Control volumes The conservation of mass and the conservation of energy principles for open systems or control volumes apply to systems having mass crossing the system boundary or control surface. In addition to the heat transfer and work crossing the system boundaries, m ...
... Conservation of Energy for Control volumes The conservation of mass and the conservation of energy principles for open systems or control volumes apply to systems having mass crossing the system boundary or control surface. In addition to the heat transfer and work crossing the system boundaries, m ...
Chapter 1 Matter and Energy Classifying Matter – An Exercise
... Practice Solutions Representations of Matter Metals can be distinguished from nonmetals by the luster and ability to conduct electricity. Since we do not know how each of elements in Figure 1.4 conduct electricity, we need to use luster as our measure. Nonmetals are usually dull, with the exception ...
... Practice Solutions Representations of Matter Metals can be distinguished from nonmetals by the luster and ability to conduct electricity. Since we do not know how each of elements in Figure 1.4 conduct electricity, we need to use luster as our measure. Nonmetals are usually dull, with the exception ...
Physical Chemistry: Thermodynamics, Structure, And Change By
... Paula. Phase diagrams 7. Chemical equilibrium PART 2: STRUCTURE 8. OUP: Berry: Physical Chemistry - Oxford University Physical Chemistry is a textbook for courses in physical chemistry structure to properties of condensed matter, then to statistical and thermodynamic Physical Chemistry, 10/e By Pete ...
... Paula. Phase diagrams 7. Chemical equilibrium PART 2: STRUCTURE 8. OUP: Berry: Physical Chemistry - Oxford University Physical Chemistry is a textbook for courses in physical chemistry structure to properties of condensed matter, then to statistical and thermodynamic Physical Chemistry, 10/e By Pete ...
Lab Activity: Determining Specific Heat of a Metal
... o In exothermic reactions, energy is _________________ so that the surroundings get _______________ and the system _______________. There is _________ energy in the system after the reaction than there was before the reaction. o In endothermic reactions, energy is _________________ so that the surro ...
... o In exothermic reactions, energy is _________________ so that the surroundings get _______________ and the system _______________. There is _________ energy in the system after the reaction than there was before the reaction. o In endothermic reactions, energy is _________________ so that the surro ...
Thermodynamics Institute of Lifelong Learning, University of Delhi
... to be reversible if it can proceed in forward and backward directions by a small change in state variables. When the system comes back to the initial state the surroundings are also restored to their initial state. That is, the process is said to be reversible only if the system can retrace its step ...
... to be reversible if it can proceed in forward and backward directions by a small change in state variables. When the system comes back to the initial state the surroundings are also restored to their initial state. That is, the process is said to be reversible only if the system can retrace its step ...
Kinetics and Equilibrium ___ 1. In a chemical reaction the use of a
... molecular collisions increases; (4) number of molecular collisions decreases. ___ 14. As 1 gram of H2O(s) changes to H2O(l), the entropy of the system (2) decreases; (3) remains the same. ___ 15. The enthalpy of a reaction may also be called the reaction; (3) entropy; (4) additivity. ...
... molecular collisions increases; (4) number of molecular collisions decreases. ___ 14. As 1 gram of H2O(s) changes to H2O(l), the entropy of the system (2) decreases; (3) remains the same. ___ 15. The enthalpy of a reaction may also be called the reaction; (3) entropy; (4) additivity. ...
Introduction to Kinetics and Equilibrium
... Introduction to Kinetics and Equilibrium Kinetics and equilibrium are two of the most important areas in chemistry Entire books and important areas in chemistry. Entire books and courses at the undergraduate and graduate level are devoted to them. Chemical kinetics – the study of the rates of ...
... Introduction to Kinetics and Equilibrium Kinetics and equilibrium are two of the most important areas in chemistry Entire books and important areas in chemistry. Entire books and courses at the undergraduate and graduate level are devoted to them. Chemical kinetics – the study of the rates of ...
boltzmann`s entropy and time`s arrow
... not require) the assumption that an isolated "aged" macroscopic system should be found in different macroscopic states M for fractions of time that equal the ratio of ir M l to the total phase space volume IFI having the same energy. Unless there are reasons to the contrary (such as extra additive c ...
... not require) the assumption that an isolated "aged" macroscopic system should be found in different macroscopic states M for fractions of time that equal the ratio of ir M l to the total phase space volume IFI having the same energy. Unless there are reasons to the contrary (such as extra additive c ...
Energy
... The greenhouse effect is a warming effect due to the presence of gases in the atmosphere which absorb infrared radiation that has reached the earth from the sun, and do not allow it to pass back into space. A limited greenhouse effect is desirable because it moderates the temperature changes in the ...
... The greenhouse effect is a warming effect due to the presence of gases in the atmosphere which absorb infrared radiation that has reached the earth from the sun, and do not allow it to pass back into space. A limited greenhouse effect is desirable because it moderates the temperature changes in the ...
Recaps and Additional Slides Chem 20B: Reference
... Energy changes with making and breaking bonds, heat Acids & bases Electrochemistry Relation to thermodynamics, energy storage Heat capacity ...
... Energy changes with making and breaking bonds, heat Acids & bases Electrochemistry Relation to thermodynamics, energy storage Heat capacity ...