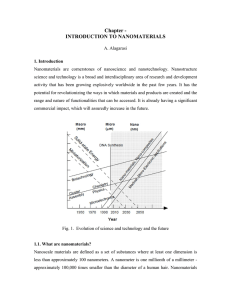
CHE 106 Chapter 5
... Heat of Formation (DH compound from elements) labeled DHf Heat of formation (DHf) is usually given for reactants and products in standard states (since DH depends on the state of these items). When in standard state, the denotation is DH°f ...
... Heat of Formation (DH compound from elements) labeled DHf Heat of formation (DHf) is usually given for reactants and products in standard states (since DH depends on the state of these items). When in standard state, the denotation is DH°f ...
06_00 AP PPT Thermochemistry
... EU 3.C – Chemical and physical transformations may be observed in several ways and typically involve a change in energy. EK 3.C.2 – Net changes in energy for a chemical reaction can be endothermic or exothermic. EU 5.A – Two systems with different temperatures that are in thermal contact will excha ...
... EU 3.C – Chemical and physical transformations may be observed in several ways and typically involve a change in energy. EK 3.C.2 – Net changes in energy for a chemical reaction can be endothermic or exothermic. EU 5.A – Two systems with different temperatures that are in thermal contact will excha ...
Presentation by class of 2013
... constant and it is omitted for calculations of the equilibrium constant in dilute aqueous solutions. If the reaction is not in aqueous solution, then the concentration of water must be included even if it is in the liquid phase; as the concentration of water can vary. However, the concentration ...
... constant and it is omitted for calculations of the equilibrium constant in dilute aqueous solutions. If the reaction is not in aqueous solution, then the concentration of water must be included even if it is in the liquid phase; as the concentration of water can vary. However, the concentration ...
chapter 13 - Humble ISD
... If the Keq < 1, then Reactants are favored If the Keq = 1, then Products and Reactants are equal If the Keq > 1 then Products are favored Since the Keq is 19.5 and 19.5 is greater then 1, more Products will be present then ...
... If the Keq < 1, then Reactants are favored If the Keq = 1, then Products and Reactants are equal If the Keq > 1 then Products are favored Since the Keq is 19.5 and 19.5 is greater then 1, more Products will be present then ...
Lecture 1 and 2a - Thermochemistry
... Larger objects have more heat capacity than smaller objects of the same composition. The units of heat capacity are J/°C or equivalently J/K. Specific heat capacity is the heat required to raise an object of one gram by one degree Celsius. Because mass is part of the definition of specific heat capa ...
... Larger objects have more heat capacity than smaller objects of the same composition. The units of heat capacity are J/°C or equivalently J/K. Specific heat capacity is the heat required to raise an object of one gram by one degree Celsius. Because mass is part of the definition of specific heat capa ...
File
... 13. Given the reaction N2(g) + 3Cl2(g) 2NCl3(g) if 1.0 mole of N2, Cl2, are placed in a 2L container and allowed to reach equilibrium. What is the concentration of NCl3 and N2 if 0.75 mole of Cl3 remains? Kc? ([NCl3]=0.083M, [N2]=0.459M, Kc=0.28) 14. Can a pressure change be used to shift the pos ...
... 13. Given the reaction N2(g) + 3Cl2(g) 2NCl3(g) if 1.0 mole of N2, Cl2, are placed in a 2L container and allowed to reach equilibrium. What is the concentration of NCl3 and N2 if 0.75 mole of Cl3 remains? Kc? ([NCl3]=0.083M, [N2]=0.459M, Kc=0.28) 14. Can a pressure change be used to shift the pos ...
- Cypress HS
... concentrations of all reactants remain constant with time. The position of equilibrium is described by a function called the equilibrium constant, Kc, which is the ratio of the amount of product present to the amount of reactant remaining once the point of equilibrium has been reached. You will dete ...
... concentrations of all reactants remain constant with time. The position of equilibrium is described by a function called the equilibrium constant, Kc, which is the ratio of the amount of product present to the amount of reactant remaining once the point of equilibrium has been reached. You will dete ...
Document
... • Knowledge of the chemical composition of the combustion products is required to determine the adiabatic flame temperature. • At typical adiabatic flame temperatures of hydrocarbons (close to 2300 K), dissociation of the combustion products occurs, and the chemical composition of the products must ...
... • Knowledge of the chemical composition of the combustion products is required to determine the adiabatic flame temperature. • At typical adiabatic flame temperatures of hydrocarbons (close to 2300 K), dissociation of the combustion products occurs, and the chemical composition of the products must ...
Lecture Notes in Physical Chemistry Semester 2: Kinetics and
... We spent last semester learning about the structure and behavior of individual molecules. This semester will be spent learning about the behavior of collections of molecules. We’ll start with gases, where the molecules more or less act individually. ...
... We spent last semester learning about the structure and behavior of individual molecules. This semester will be spent learning about the behavior of collections of molecules. We’ll start with gases, where the molecules more or less act individually. ...
In_Class_Practice Chapter 17 PreAP
... 6. Use Le Châtelier’s principle to predict how each of the following changes would affect this equilibrium. C2H4O(g) CH4(g) + CO(g) a. adding CH4(g) to the system b. removing CO(g) from the system c. removing C2H4O(g) from the system 7. How would decreasing the volume of the reaction vessel affect ...
... 6. Use Le Châtelier’s principle to predict how each of the following changes would affect this equilibrium. C2H4O(g) CH4(g) + CO(g) a. adding CH4(g) to the system b. removing CO(g) from the system c. removing C2H4O(g) from the system 7. How would decreasing the volume of the reaction vessel affect ...
5 Energetics/thermochemistry
... Because of these random motions at all temperatures above absolute zero (0 K or −273 °C), the particles in matter have kinetic energy. The average kinetic energy of these individual particles causes what we perceive through sense perception as warmth. Whenever a substance becomes warmer, the average ...
... Because of these random motions at all temperatures above absolute zero (0 K or −273 °C), the particles in matter have kinetic energy. The average kinetic energy of these individual particles causes what we perceive through sense perception as warmth. Whenever a substance becomes warmer, the average ...
Document
... • Thermodynamics lets us predict whether a process will occur but NOT how fast it will happen • A spontaneous process is one that occurs without outside intervention. As long as the activation energy is supplied, the reaction will proceed forward. ...
... • Thermodynamics lets us predict whether a process will occur but NOT how fast it will happen • A spontaneous process is one that occurs without outside intervention. As long as the activation energy is supplied, the reaction will proceed forward. ...
Electrical theory of thermodynamics and particle scale heat engines
... the hot particles individually to the load system or circuit, and of effectively “rectifying” the random motions of the hot particles in the process. While the first problem would be addressed by using electromagnetic fields as the coupling mechanism in place of a mechanical piston or turbine, the s ...
... the hot particles individually to the load system or circuit, and of effectively “rectifying” the random motions of the hot particles in the process. While the first problem would be addressed by using electromagnetic fields as the coupling mechanism in place of a mechanical piston or turbine, the s ...