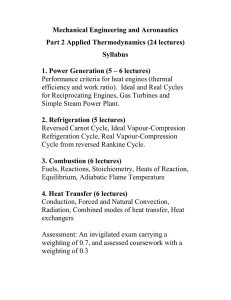
Mechanical Engineering and Aeronautics
... the beginning of the compression process, air is at 100 kPa, and 30oC, and 840 kJ/kg of heat is transferred to air during the constant volume heat addition process. Taking into account the variation of specific heats with temperature, determine (a) the pressure and temperature at the end of the heat ...
... the beginning of the compression process, air is at 100 kPa, and 30oC, and 840 kJ/kg of heat is transferred to air during the constant volume heat addition process. Taking into account the variation of specific heats with temperature, determine (a) the pressure and temperature at the end of the heat ...
Thermodynamics and Equilibrium
... Standard Entropies and the Third Law of Thermodynamics • The third law of thermodynamics states that a substance that is perfectly crystalline at 0 K has an entropy of zero. – When temperature is raised, however, the substance becomes more disordered as it absorbs heat. – The entropy of a substance ...
... Standard Entropies and the Third Law of Thermodynamics • The third law of thermodynamics states that a substance that is perfectly crystalline at 0 K has an entropy of zero. – When temperature is raised, however, the substance becomes more disordered as it absorbs heat. – The entropy of a substance ...
In Class Problems and Notes AP Chemistry General Equilibrium
... need more reactants to make the reactions occur at an equal rate. Since you will have more reactants at equilibrium, Keq will be less than one. Case III: In this case the activation energies for the forward and the reverse reactions are about the same, therefore you would expect approximately equal ...
... need more reactants to make the reactions occur at an equal rate. Since you will have more reactants at equilibrium, Keq will be less than one. Case III: In this case the activation energies for the forward and the reverse reactions are about the same, therefore you would expect approximately equal ...
mean-field approach to magnetism
... where in case of pure mechanical interactions the V (volume), T (temperature) and N (particle number) are fixed. This means that we consider a closed system in contact with a heat-bath at temperature T. Due to the heat-exchange with the heat reservoir the energy can fluctuate. We learn from thermody ...
... where in case of pure mechanical interactions the V (volume), T (temperature) and N (particle number) are fixed. This means that we consider a closed system in contact with a heat-bath at temperature T. Due to the heat-exchange with the heat reservoir the energy can fluctuate. We learn from thermody ...
Practical Exercises in Physical Chemistry
... chemical species. The underlying idea of this method is to follow the evolution in time of a chemical system from a non-equilibrium state into equilibrium. The development of some of these experimental methods was awarded by the Nobel Prize in chemistry 1967 (M. Eigen, R. G. W. Norrish and G. Porter ...
... chemical species. The underlying idea of this method is to follow the evolution in time of a chemical system from a non-equilibrium state into equilibrium. The development of some of these experimental methods was awarded by the Nobel Prize in chemistry 1967 (M. Eigen, R. G. W. Norrish and G. Porter ...
THE FREE ENERGIES OF FORMATION OF AQUEOUS d
... We shall deal with the general question in future publications. For the present it is profitable to assumethat organisms obey the second law of thermodynamics as do macroscopic systems. We shall assume, therefore, that the theoretical maximum amount of work derivable from any process is given by the ...
... We shall deal with the general question in future publications. For the present it is profitable to assumethat organisms obey the second law of thermodynamics as do macroscopic systems. We shall assume, therefore, that the theoretical maximum amount of work derivable from any process is given by the ...
Chapter 10
... State functions are properties that are determined by the state of the system, regardless of how that condition was achieved. ...
... State functions are properties that are determined by the state of the system, regardless of how that condition was achieved. ...
Thermal Energy
... from the hotter object to the colder object until eventually the two objects reach the same temperature. When this common final temperature is reached, the two objects are said to be in thermal equilibrium. As long as they are isolated from other objects and cannot exchange any heat with their surr ...
... from the hotter object to the colder object until eventually the two objects reach the same temperature. When this common final temperature is reached, the two objects are said to be in thermal equilibrium. As long as they are isolated from other objects and cannot exchange any heat with their surr ...
File
... Chemical Reactions - Chemical reactivity and products of chemical reaction Significant figures/Mental Math Mixtures - just stoich/reaction problems in disguise Gas Laws – simple calculations, root-mean-square speed, nonideal behavior Electrochemistry – not on the review, but you should remember it s ...
... Chemical Reactions - Chemical reactivity and products of chemical reaction Significant figures/Mental Math Mixtures - just stoich/reaction problems in disguise Gas Laws – simple calculations, root-mean-square speed, nonideal behavior Electrochemistry – not on the review, but you should remember it s ...
M.Sc. PHYSICAL CHEMISTRY
... (i) To learn about the classical mechanics of thermodynamics (ii) To understand and appreciate the concepts of statistical thermodynamics (iii) To apply the concepts of statistical thermodynamics for the study of equilibrium reactions and reaction rates UNIT I Chemical Thermodynamics-I First law of ...
... (i) To learn about the classical mechanics of thermodynamics (ii) To understand and appreciate the concepts of statistical thermodynamics (iii) To apply the concepts of statistical thermodynamics for the study of equilibrium reactions and reaction rates UNIT I Chemical Thermodynamics-I First law of ...
الشريحة 1
... Sample Exercise: Describing and Calculating Energy Changes A bowler lifts a 5.4-kg (12-lb) bowling ball from ground level to a height of 1.6 m (5.2 feet) and then drops the ball back to the ground. (a) What happens to the potential energy of the bowling ball as it is raised from the ground? (b) Wha ...
... Sample Exercise: Describing and Calculating Energy Changes A bowler lifts a 5.4-kg (12-lb) bowling ball from ground level to a height of 1.6 m (5.2 feet) and then drops the ball back to the ground. (a) What happens to the potential energy of the bowling ball as it is raised from the ground? (b) Wha ...
















![[PDF]](http://s1.studyres.com/store/data/008813344_1-6b54197619c7ffbc0fcfc4fbb7e270fc-300x300.png)






