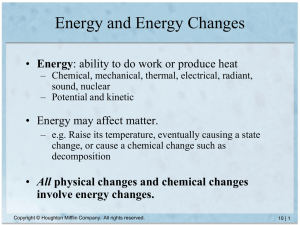
Energy can change forms but is never lost.
... The chemical energy in fossil fuels is converted into other forms of energy for specific uses. In power plants, people burn coal to convert its chemical energy into electrical energy. In homes, people burn natural gas to convert its chemical energy into heat that warms them and cooks their food. In ...
... The chemical energy in fossil fuels is converted into other forms of energy for specific uses. In power plants, people burn coal to convert its chemical energy into electrical energy. In homes, people burn natural gas to convert its chemical energy into heat that warms them and cooks their food. In ...
Grade 8 Model Science Unit 5: Relationships among Forms of... Instructional Days: 20 Unit Summary
... ball versus a tennis ball. Through using one of these examples, students can record either mass or speed data to identify linear and nonlinear relationships. When constructing and interpreting graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to ...
... ball versus a tennis ball. Through using one of these examples, students can record either mass or speed data to identify linear and nonlinear relationships. When constructing and interpreting graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to ...
Grade 8 Model Science Unit 5
... ball versus a tennis ball. Through using one of these examples, students can record either mass or speed data to identify linear and nonlinear relationships. When constructing and interpreting graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to ...
... ball versus a tennis ball. Through using one of these examples, students can record either mass or speed data to identify linear and nonlinear relationships. When constructing and interpreting graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to ...
Energy: Forms and Changes
... Roller coasters work because of the energy that is built into the system. Initially, the cars are pulled mechanically up the tallest hill, giving them a great deal of potential energy. From that point, the conversion between potential and kinetic energy powers the cars throughout the entire ride. ...
... Roller coasters work because of the energy that is built into the system. Initially, the cars are pulled mechanically up the tallest hill, giving them a great deal of potential energy. From that point, the conversion between potential and kinetic energy powers the cars throughout the entire ride. ...
Air Car points - Beavercreek City Schools
... 9V battery clip 19th One wire lead with two alligator clips Four wheels 1 3/8” diameter with 3/32” tread width Two 9” length of 1/8” diameter dowel rod. Days to turn in Four straws 9/64” diameter X 5” length. completed (Some replacement parts are available for purchase at school if neede ...
... 9V battery clip 19th One wire lead with two alligator clips Four wheels 1 3/8” diameter with 3/32” tread width Two 9” length of 1/8” diameter dowel rod. Days to turn in Four straws 9/64” diameter X 5” length. completed (Some replacement parts are available for purchase at school if neede ...
Q4 U2 Energy and Chemical Reactions
... Bond Enthalpy is the energy required to break a chemical bond. It is usually expressed in units of kJ/mol ΔH = ∑ ΔH(bonds broken) - ∑ ΔH(bonds formed) Add up all the energies of the broken bonds; add up all the energies of the bonds that are reformed and subtract one from the other. ...
... Bond Enthalpy is the energy required to break a chemical bond. It is usually expressed in units of kJ/mol ΔH = ∑ ΔH(bonds broken) - ∑ ΔH(bonds formed) Add up all the energies of the broken bonds; add up all the energies of the bonds that are reformed and subtract one from the other. ...
Energy - Images
... It can only be converted from one form to another. If energy seems to disappear, then scientists look for it – leading to many ...
... It can only be converted from one form to another. If energy seems to disappear, then scientists look for it – leading to many ...
Energy - Kawameeh Middle School
... • An open system is a system that exchanges matter or energy with the environment. • A closed system is a system that does not exchange matter or energy with the environment. • In reality, there are no closed systems because every physical system transfers some energy to or from its environment. ...
... • An open system is a system that exchanges matter or energy with the environment. • A closed system is a system that does not exchange matter or energy with the environment. • In reality, there are no closed systems because every physical system transfers some energy to or from its environment. ...
From the first law of thermodynamics
... Foods and Fuels Foods •Fuel value = energy released when 1 g of substance is burned. •1 nutritional Calorie, 1 Cal = 1000 cal = 1 kcal. •Energy in our bodies comes from carbohydrates and fats (mostly). •Intestines: carbohydrates converted into glucose: C6H12O6 + 6O2 6CO2 + 6H2O, DH = -2816 kJ ...
... Foods and Fuels Foods •Fuel value = energy released when 1 g of substance is burned. •1 nutritional Calorie, 1 Cal = 1000 cal = 1 kcal. •Energy in our bodies comes from carbohydrates and fats (mostly). •Intestines: carbohydrates converted into glucose: C6H12O6 + 6O2 6CO2 + 6H2O, DH = -2816 kJ ...
Kinetic energy - Claseshistoria.com
... The sources of energy can be non-renewable or renewable. The non-renewable sources are limited and cannot be replaced when they run out while the renewable sources can be replaced or used again and they will not run out, at least for now. The main sources are non- renewable, in particular, fossil fu ...
... The sources of energy can be non-renewable or renewable. The non-renewable sources are limited and cannot be replaced when they run out while the renewable sources can be replaced or used again and they will not run out, at least for now. The main sources are non- renewable, in particular, fossil fu ...
Chapter 10
... stated as “the energy of the universe is constant.” • Internal Energy (E) = kinetic energy + potential energy • ΔE = q + w = change in internal energy q = heat absorbed by the system w = work done on the system Copyright © Houghton Mifflin Company. All rights reserved. ...
... stated as “the energy of the universe is constant.” • Internal Energy (E) = kinetic energy + potential energy • ΔE = q + w = change in internal energy q = heat absorbed by the system w = work done on the system Copyright © Houghton Mifflin Company. All rights reserved. ...
Energy - Glow Blogs
... Even systems that are designed to produce heat will have some energy losses. For example, the element of a kettle is designed to heat up water, but not all of the energy will go into heating up the water. Some of the energy is used to heat up the kettle; some heat will be ‘lost’ to the room, etc. Si ...
... Even systems that are designed to produce heat will have some energy losses. For example, the element of a kettle is designed to heat up water, but not all of the energy will go into heating up the water. Some of the energy is used to heat up the kettle; some heat will be ‘lost’ to the room, etc. Si ...
Chapter 11A 4-7 - WVU Plasma Physics
... Energy flows from higher temp. to lower temp. (0th law) Rate of energy transfer (P=power) depends on – Temperature difference (TH-TC) – Area of contact (A) – Thermal conductivity of the material (k) ...
... Energy flows from higher temp. to lower temp. (0th law) Rate of energy transfer (P=power) depends on – Temperature difference (TH-TC) – Area of contact (A) – Thermal conductivity of the material (k) ...
Energy and Energy Resources Energy Transformations
... Have you ever made popcorn in a microwave oven? Energy changes form when you make popcorn, as shown below. A microwave oven changes electric energy into radiant energy. Radiant energy changes into thermal energy in the popcorn kernels. Thermal energy causes water in the popcorn kernels to turn to st ...
... Have you ever made popcorn in a microwave oven? Energy changes form when you make popcorn, as shown below. A microwave oven changes electric energy into radiant energy. Radiant energy changes into thermal energy in the popcorn kernels. Thermal energy causes water in the popcorn kernels to turn to st ...
RP 3P3 Energy Transfer - NC Science Wiki
... prevented completely. Therefore the total amount of energy available for transformation is almost always decreasing. For example, almost all of the energy stored in the molecules of gasoline used during an automobile trip goes, by way of friction and exhaust, into producing a slightly warmer car, ro ...
... prevented completely. Therefore the total amount of energy available for transformation is almost always decreasing. For example, almost all of the energy stored in the molecules of gasoline used during an automobile trip goes, by way of friction and exhaust, into producing a slightly warmer car, ro ...
Station 2: Kinetic Energy
... Plants used light energy from the Sun for photosynthesis to make their chemicals. This stored chemical energy was transferred to stored chemical energy in animals that ate the plants. When the living things died, they were gradually buried by layers of rock. The buried remains were put under pressur ...
... Plants used light energy from the Sun for photosynthesis to make their chemicals. This stored chemical energy was transferred to stored chemical energy in animals that ate the plants. When the living things died, they were gradually buried by layers of rock. The buried remains were put under pressur ...
University Physics 226N/231N Old Dominion University Friction
... § We define work W as net force applied times the distance its applied in • The force can be different at various spots so we really need to add together (roughly constant) force over small distances • This is really another integral, like impulse. It’s a scaler (units: 1 J=1 N-m) ...
... § We define work W as net force applied times the distance its applied in • The force can be different at various spots so we really need to add together (roughly constant) force over small distances • This is really another integral, like impulse. It’s a scaler (units: 1 J=1 N-m) ...
What is Energy?
... Sound is a type of energy made by vibrations. When any object vibrates, it causes movement in the air particles. These particles bump into the particles close to them, which makes them vibrate too causing them to bump into more air particles. This movement, called sound waves, keeps going unt ...
... Sound is a type of energy made by vibrations. When any object vibrates, it causes movement in the air particles. These particles bump into the particles close to them, which makes them vibrate too causing them to bump into more air particles. This movement, called sound waves, keeps going unt ...
CLASS IX work and energy
... Previous Years’ Questions 1. Define work, energy and power. Give the SI (iii) An object of mass 10 kg is at a certain height units for each of the these quantities. A man above the ground. If the potential energy of whose mass is 80 kg climbs up 30 steps of the the object is 400 J, find the heig ...
... Previous Years’ Questions 1. Define work, energy and power. Give the SI (iii) An object of mass 10 kg is at a certain height units for each of the these quantities. A man above the ground. If the potential energy of whose mass is 80 kg climbs up 30 steps of the the object is 400 J, find the heig ...
X-Ray Production & Emission
... A projectile e- that completely avoids the orbital e- as it passes through a target atom may pass close enough to the nucleus of the atom to convert some of the projectile e- kinetic energy to EM energy ...
... A projectile e- that completely avoids the orbital e- as it passes through a target atom may pass close enough to the nucleus of the atom to convert some of the projectile e- kinetic energy to EM energy ...
Energy - Clover Park School District
... between subsystems and stored in various ways within the system, the total energy of a system changes only by the amount of energy transferred into and out of the system. At the macroscopic scale, energy manifests itself in multiple phenomena, such as motion, light, sound, electrical and magnetic fi ...
... between subsystems and stored in various ways within the system, the total energy of a system changes only by the amount of energy transferred into and out of the system. At the macroscopic scale, energy manifests itself in multiple phenomena, such as motion, light, sound, electrical and magnetic fi ...
ExamView Pro - science 2nd 9 weeks.tst
... 1. A wave travels through a medium because a. the medium’s particles are carried along with the wave. b. the wave’s energy passes from particle to particle. c. the medium transfers electromagnetic energy. d. the wave increases the potential energy of its medium. 2. Refraction occurs when a wave a. e ...
... 1. A wave travels through a medium because a. the medium’s particles are carried along with the wave. b. the wave’s energy passes from particle to particle. c. the medium transfers electromagnetic energy. d. the wave increases the potential energy of its medium. 2. Refraction occurs when a wave a. e ...
Energy
... • Energy can not be created or destroyed. • Whenever a moving object experiences friction, some of its kinetic energy is transformed to thermal energy this shows that energy is not destroyed, just transformed. • Einstein discovered that matter can be transformed to energy. • Destroying a small amoun ...
... • Energy can not be created or destroyed. • Whenever a moving object experiences friction, some of its kinetic energy is transformed to thermal energy this shows that energy is not destroyed, just transformed. • Einstein discovered that matter can be transformed to energy. • Destroying a small amoun ...
... Sound is a type of energy made by vibrations. When any object vibrates, it causes movement in the air particles. These particles bump into the particles close to them, which makes them vibrate too causing them to bump into more air particles. This movement, called sound waves, keeps going until they ...