Photosynthesis Pt 1 Light
... • A pigment is a substance that absorbs particular wavelengths of light. • The colors that you see are the wavelengths that are reflected, not absorbed. • If chlorophyll is a green pigment, then what wavelength is not being absorbed? • GREEN ...
... • A pigment is a substance that absorbs particular wavelengths of light. • The colors that you see are the wavelengths that are reflected, not absorbed. • If chlorophyll is a green pigment, then what wavelength is not being absorbed? • GREEN ...
Modern Atomic Theory (aka the electron chapter!)
... e- can only exist in certain discrete orbits e- is restricted to QUANTIZED energy state (quanta = bundles of energy) ...
... e- can only exist in certain discrete orbits e- is restricted to QUANTIZED energy state (quanta = bundles of energy) ...
3/27 Lecture Slides
... at a frequency of 750 MHz. This is in the radio frequency and Hz = s-1. What is the wavelength of this light? An infrared absorption band occurs at a wavenumber of 812 cm-1. What is the wavelength (in mm) and energy (J/photon) of that light? What type of light involves transitions of inner shell ele ...
... at a frequency of 750 MHz. This is in the radio frequency and Hz = s-1. What is the wavelength of this light? An infrared absorption band occurs at a wavenumber of 812 cm-1. What is the wavelength (in mm) and energy (J/photon) of that light? What type of light involves transitions of inner shell ele ...
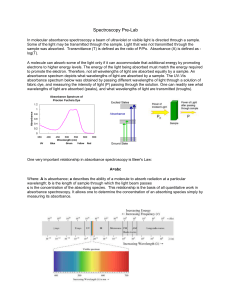
1. a) 25% b)86% 2. For my opinion, I think the way to make
... 1. a) 25% b)86% 2. For my opinion, I think the way to make correction for background absorption caused by sample matrices is require storage of a reference spectrum from a biomembrane before the interaction with a substrate. Alteration of this membrane caused by external influence are then stored in ...
... 1. a) 25% b)86% 2. For my opinion, I think the way to make correction for background absorption caused by sample matrices is require storage of a reference spectrum from a biomembrane before the interaction with a substrate. Alteration of this membrane caused by external influence are then stored in ...
Spectrometry 1 R
... The study of how the chemical compound interacts with different wavelengths in a given region of electromagnetic radiation is called spectroscopy or spectrochemical analysis. The collection of measurements signals (absorbance) of the compound as a function of electromagnetic radiation is called a sp ...
... The study of how the chemical compound interacts with different wavelengths in a given region of electromagnetic radiation is called spectroscopy or spectrochemical analysis. The collection of measurements signals (absorbance) of the compound as a function of electromagnetic radiation is called a sp ...
Extreme Sensitivity in Photoacoustics by Using Optical Cantilever
... ideal trace gas measurement system has to fulfill various requirements: ...
... ideal trace gas measurement system has to fulfill various requirements: ...
Conference title, upper and lower case, bolded, 18 point type
... The development of compact ICL based trace gas sensors will permit the targeting of strong fundamental rotationalvibrational transitions in the mid-infrared which are one to two orders of magnitude more intense than transitions in overtone and combination bands in the near-infrared. Specifically, th ...
... The development of compact ICL based trace gas sensors will permit the targeting of strong fundamental rotationalvibrational transitions in the mid-infrared which are one to two orders of magnitude more intense than transitions in overtone and combination bands in the near-infrared. Specifically, th ...
Spectrophotometry Chapter 18
... • The electrons of an atom have different energies. • Not all energies exist, only certain allowed energy levels. • Electrons with more energy are able to get farther away from the nucleus and its + charges. • Therefore, electrons in higher energy levels spend more time farther away from the nucleus ...
... • The electrons of an atom have different energies. • Not all energies exist, only certain allowed energy levels. • Electrons with more energy are able to get farther away from the nucleus and its + charges. • Therefore, electrons in higher energy levels spend more time farther away from the nucleus ...
Háskóli Íslands Raunvísindadeild,
... Fig. 1. Equipments for recording emission spectra.. The equipment consists of (Fig. 1): –a monochromator (í: Ljósgreiða; see Figure)) (sjá nánar neðar) –a photomultiplier (í: Ljósmagnari)) for converging light signal to electric signal. –a high voltage Power supply for the photomultiplier. –an integ ...
... Fig. 1. Equipments for recording emission spectra.. The equipment consists of (Fig. 1): –a monochromator (í: Ljósgreiða; see Figure)) (sjá nánar neðar) –a photomultiplier (í: Ljósmagnari)) for converging light signal to electric signal. –a high voltage Power supply for the photomultiplier. –an integ ...
Preview of Period 3: Electromagnetic Waves – Radiant Energy II
... Preview of Period 3: Electromagnetic Waves – Radiant Energy II ...
... Preview of Period 3: Electromagnetic Waves – Radiant Energy II ...
5.33 Lecture Notes: Introduction to Spectroscopy
... With light, you aren’t looking directly at the molecule—the matter—but its “ghost.” You observe the light’s interaction with different degrees of freedom of the molecule. Each type of spectroscopy—different light frequency—gives a different picture → the spectrum. Spectroscopy is a general methodolo ...
... With light, you aren’t looking directly at the molecule—the matter—but its “ghost.” You observe the light’s interaction with different degrees of freedom of the molecule. Each type of spectroscopy—different light frequency—gives a different picture → the spectrum. Spectroscopy is a general methodolo ...
About UV-Vis Molecular Absorbance Spectroscopy
... In molecular absorbance spectroscopy a beam of ultraviolet or visible light is directed through a sample. Some of the light may be transmitted through the sample. Light that was not transmitted through the sample was absorbed. Transmittance (T) is defined as the ratio of P/Po. Absorbance (A) is defi ...
... In molecular absorbance spectroscopy a beam of ultraviolet or visible light is directed through a sample. Some of the light may be transmitted through the sample. Light that was not transmitted through the sample was absorbed. Transmittance (T) is defined as the ratio of P/Po. Absorbance (A) is defi ...
Modeling, Simulation and Application of the Probe Beam Deflection
... simulation in combination with an acoustic wave simulation will allow for the prediction of beam deflection or refraction for various experimental topologies and implementations. ...
... simulation in combination with an acoustic wave simulation will allow for the prediction of beam deflection or refraction for various experimental topologies and implementations. ...
5th Grade Energy Study Guide
... 1. ________________ Back and forth motion. (This is what causes sounds to occur). 2. ________________ An area of bunched-up and spread-out particles that move outward in all directions. 3. ________________ A sound wave must have this to travel. (Example- Solid, Liquid, or Gas.) 4. ________________ T ...
... 1. ________________ Back and forth motion. (This is what causes sounds to occur). 2. ________________ An area of bunched-up and spread-out particles that move outward in all directions. 3. ________________ A sound wave must have this to travel. (Example- Solid, Liquid, or Gas.) 4. ________________ T ...
Arrangement of Electrons in Atoms
... Called the dual wave-particle nature of light! Electromagnetic Radiation - form of E that exhibits wavelike behavior as it travels thru space Electromagnetic Spectrum - all of the forms of electromagnetic radiation (visible light, x-rays, uv and infrared light, micro and radio waves) ...
... Called the dual wave-particle nature of light! Electromagnetic Radiation - form of E that exhibits wavelike behavior as it travels thru space Electromagnetic Spectrum - all of the forms of electromagnetic radiation (visible light, x-rays, uv and infrared light, micro and radio waves) ...
tire
... 13. Semiconductor chip that has replaced the photographic plate for recording astronomical images on a telescope. Also known as charge coupled device. 14. A form of energy that consists of oscillating electric and magnetic fields that travels through space at the speed of light. 15. The acronym used ...
... 13. Semiconductor chip that has replaced the photographic plate for recording astronomical images on a telescope. Also known as charge coupled device. 14. A form of energy that consists of oscillating electric and magnetic fields that travels through space at the speed of light. 15. The acronym used ...
Photosynthesis has 3 stages
... appear (r)_________________. In the fall the leaves turn many different colors. This is because there is another pigment found in them. This pigment is called (s)________________. Having both kinds of pigments allows plants to (t) ________________ more light energy. Production of oxygen: Disk shaped ...
... appear (r)_________________. In the fall the leaves turn many different colors. This is because there is another pigment found in them. This pigment is called (s)________________. Having both kinds of pigments allows plants to (t) ________________ more light energy. Production of oxygen: Disk shaped ...
Fourier Transform IR Spectroscopy
... • FT – IR can take wavelength readings across the whole IR region simultaneously and smoothly, making this a very rapid technique. • The technique is non-invasive and non-destructive. Its resolution of .125 cm-1 is not spectacular in comparison to other vibrational techniques and it will not give th ...
... • FT – IR can take wavelength readings across the whole IR region simultaneously and smoothly, making this a very rapid technique. • The technique is non-invasive and non-destructive. Its resolution of .125 cm-1 is not spectacular in comparison to other vibrational techniques and it will not give th ...
Photosynthesis in plants requires sunlight in addition
... spectral coverage is severely limited. Constructing a suitable LED-based plant grow light that adequately covers the high reaction yield portions of the photosynthesis absorption spectrum thus requires a number of LEDs emitting at different wavelengths. Electrospell’s broadband SpectrafillTM LEDs ge ...
... spectral coverage is severely limited. Constructing a suitable LED-based plant grow light that adequately covers the high reaction yield portions of the photosynthesis absorption spectrum thus requires a number of LEDs emitting at different wavelengths. Electrospell’s broadband SpectrafillTM LEDs ge ...
At what intensity is the laser set?
... Though Einstein is most famous for his work in describing relativity in mechanics, his Nobel Prize was for understanding a very simple experiment. It was long understood that if you directed light of a certain wavelength at a piece of metal, it would emit electrons. In classical theory, the energy o ...
... Though Einstein is most famous for his work in describing relativity in mechanics, his Nobel Prize was for understanding a very simple experiment. It was long understood that if you directed light of a certain wavelength at a piece of metal, it would emit electrons. In classical theory, the energy o ...
High Resolution Biomedical Imaging with Light and Sound
... illuminates tissue, where optical absorption and transient thermal expansion leads to ultrasound emission. Image contrast is based on the naturally occurring (endogenous) optical absorption in tissue. Spatial resolution and penetration depth are determined by the ultrasonic properties of tissue. Per ...
... illuminates tissue, where optical absorption and transient thermal expansion leads to ultrasound emission. Image contrast is based on the naturally occurring (endogenous) optical absorption in tissue. Spatial resolution and penetration depth are determined by the ultrasonic properties of tissue. Per ...
Argos TM - Lockheed Martin
... Figure 1: The QEPAS tuning-fork sensor. With QEPAS, researchers measure the amplitude of the sound wave that is generated when gas molecules absorb modulated light. The detection module is a low-cost, compact, quartz tuning fork, such as those commonly found in digital watches. The QEPAS sensor has ...
... Figure 1: The QEPAS tuning-fork sensor. With QEPAS, researchers measure the amplitude of the sound wave that is generated when gas molecules absorb modulated light. The detection module is a low-cost, compact, quartz tuning fork, such as those commonly found in digital watches. The QEPAS sensor has ...