The Buffer Equation
... is momentarily disturbed because the acetate ion supplied by the salt increases the [Ac-] term in the numerator. To reestablish the constant Ka at 1.75 × 10-5, the hydrogen ion term in the numerator [H3O+] is instantaneously decreased, with a corresponding increase in [HAc]. Therefore, the constant ...
... is momentarily disturbed because the acetate ion supplied by the salt increases the [Ac-] term in the numerator. To reestablish the constant Ka at 1.75 × 10-5, the hydrogen ion term in the numerator [H3O+] is instantaneously decreased, with a corresponding increase in [HAc]. Therefore, the constant ...
chp0-Intro
... • Reactions do not always proceed completely from reactants to products • Chemical equilibrium rates of forward and reverse reaction are equal e.g. ...
... • Reactions do not always proceed completely from reactants to products • Chemical equilibrium rates of forward and reverse reaction are equal e.g. ...
Reaction Kinetics. The Bromination of Acetone
... Determination of the B constant: The constant B of equation [10] is determined by measuring the absorbance of at least three solutions of known bromine concentration. At room temperature, prepare one solution by pipetting 10.0 ml of stock 0.02 M Br2 into a clean 125-ml Erlenmeyer flask. Add 10.0 ml ...
... Determination of the B constant: The constant B of equation [10] is determined by measuring the absorbance of at least three solutions of known bromine concentration. At room temperature, prepare one solution by pipetting 10.0 ml of stock 0.02 M Br2 into a clean 125-ml Erlenmeyer flask. Add 10.0 ml ...
Chemistry Review
... Fluids – gases and liquids, flow Ideal gas – imaginary gas that fits all the assumptions of the kinetic molecular theory Kelvin – SI unit of temperature Kinetic Theory- group of ideas explaining the interaction of matter and energy due to particle motion Melting – change in state from a solid to a l ...
... Fluids – gases and liquids, flow Ideal gas – imaginary gas that fits all the assumptions of the kinetic molecular theory Kelvin – SI unit of temperature Kinetic Theory- group of ideas explaining the interaction of matter and energy due to particle motion Melting – change in state from a solid to a l ...
AP Chemistry—Chapter 15: Applications of Aqueous Equilibria
... (a) The amount of acetylsalicylic acid in a single aspirin tablet is 325 mg, yet the tablet has a mass of 2.00 g. Calculate the mass percent of acetylsalicylic acid in the tablet. (b) The elements contained in acetylsalicylic acid are hydrogen, carbon, and oxygen. The combustion of 3.000 g of the pu ...
... (a) The amount of acetylsalicylic acid in a single aspirin tablet is 325 mg, yet the tablet has a mass of 2.00 g. Calculate the mass percent of acetylsalicylic acid in the tablet. (b) The elements contained in acetylsalicylic acid are hydrogen, carbon, and oxygen. The combustion of 3.000 g of the pu ...
Ch. 10: Acid-Base Titrations
... ΔpH/ΔV, of the titration curve. (c) The second derivative, Δ(ΔpH/ ΔV)/ΔV, which is the derivative of the curve in panel b. End points are taken as maxima in the derivative curve and zero crossings of the second derivative. ...
... ΔpH/ΔV, of the titration curve. (c) The second derivative, Δ(ΔpH/ ΔV)/ΔV, which is the derivative of the curve in panel b. End points are taken as maxima in the derivative curve and zero crossings of the second derivative. ...
Lewis Acids and Bases - Screenshot for timg.co.il
... • Certain metal hydroxides, insoluble in water, are amphoteric; they will react with both strong acids and strong bases. • Al(OH)3, Zn(OH)2, and Cr(OH)3 are amphoteric. ...
... • Certain metal hydroxides, insoluble in water, are amphoteric; they will react with both strong acids and strong bases. • Al(OH)3, Zn(OH)2, and Cr(OH)3 are amphoteric. ...
Homework Exercises
... 2. What happens to the rate of a reaction if the concentration of the reactants is decreased? ...
... 2. What happens to the rate of a reaction if the concentration of the reactants is decreased? ...
Unit 3 Notes
... If the temperature is increased the equilibrium will favour the .................... reaction because that will lower the temperature. The equilibrium move to the ....................., therefore the solution becomes ...................... in colour. If the temperature is reduced the equilibrium wil ...
... If the temperature is increased the equilibrium will favour the .................... reaction because that will lower the temperature. The equilibrium move to the ....................., therefore the solution becomes ...................... in colour. If the temperature is reduced the equilibrium wil ...
Acids, Bases, and Buffers
... The function of a buffer can be examined using LeChatelier’s Principle. When a strong acid is added to a buffer solution it ionizes completely forming H3O+. The H3O+ produced from the strong acid becomes part of the equilibrium. The concentration of H3O+ in the equilibrium reaction has been increase ...
... The function of a buffer can be examined using LeChatelier’s Principle. When a strong acid is added to a buffer solution it ionizes completely forming H3O+. The H3O+ produced from the strong acid becomes part of the equilibrium. The concentration of H3O+ in the equilibrium reaction has been increase ...
Supporting Information - Royal Society of Chemistry
... where Λ 0 is the molar conductivity at infinite dilution and K is a coefficient related to the electrolyte theory. A linear decrease in molar conductivity was observed up to [SDS] = 7.8×10–3 M, corresponding to the CMC value. Light scattering: Aggregation of surfactant monomers is also revealed by a ...
... where Λ 0 is the molar conductivity at infinite dilution and K is a coefficient related to the electrolyte theory. A linear decrease in molar conductivity was observed up to [SDS] = 7.8×10–3 M, corresponding to the CMC value. Light scattering: Aggregation of surfactant monomers is also revealed by a ...
Chemical Reactions.
... (copper→ Cu, sulfur→ S, oxygen→ O) subscript numbers appear after the atomic symbol and describe the number of atoms in the compound (1 copper, 1 sulfur, 4 oxygen) subscript letters describe the physical state of the compound: s = solid, l = liquid, g = gas, aq = aqueous! ...
... (copper→ Cu, sulfur→ S, oxygen→ O) subscript numbers appear after the atomic symbol and describe the number of atoms in the compound (1 copper, 1 sulfur, 4 oxygen) subscript letters describe the physical state of the compound: s = solid, l = liquid, g = gas, aq = aqueous! ...
Section 11.1 Assessment How many mole ratios can be written for
... equation, how many of each (formula units, molecules and/or atoms)? Moles: from the balanced equation, how many of each? Mass: from the balanced equation, convert known mole quantities to mass of products total & compare to mass of reactants total, should be equal. ...
... equation, how many of each (formula units, molecules and/or atoms)? Moles: from the balanced equation, how many of each? Mass: from the balanced equation, convert known mole quantities to mass of products total & compare to mass of reactants total, should be equal. ...
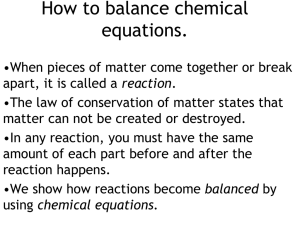
How to balance chemical equations.
... equations. •Multiply the number of atoms you have by coefficients to balance equations. •Multiply coefficients by every subscript until you hit a + or a . •Never, ever, EVER change a subscript. •Make a list of how much of each atom you have and change it as you add coefficients to your equation. •I ...
... equations. •Multiply the number of atoms you have by coefficients to balance equations. •Multiply coefficients by every subscript until you hit a + or a . •Never, ever, EVER change a subscript. •Make a list of how much of each atom you have and change it as you add coefficients to your equation. •I ...
CHEM 435 Physical Chemistry Laboratory Spring Semester, 2017
... Mastering your writing skills, particularly on the subject of scientific research, is imperative for advancing your further carriers in a science-related field. It is one of the topics that we will be working on throughout this class and will be linked to perfecting your written reports. Each writte ...
... Mastering your writing skills, particularly on the subject of scientific research, is imperative for advancing your further carriers in a science-related field. It is one of the topics that we will be working on throughout this class and will be linked to perfecting your written reports. Each writte ...
Review Material
... Relative Sizes of Ions For the Representative (s-block and p-block) Elements that form positive ions (cations), the radius of the positive ion will always be smaller than the radius of the corresponding atom. This is due primarily to the fact that when these elements form ions the outermost shell (h ...
... Relative Sizes of Ions For the Representative (s-block and p-block) Elements that form positive ions (cations), the radius of the positive ion will always be smaller than the radius of the corresponding atom. This is due primarily to the fact that when these elements form ions the outermost shell (h ...