A.P. Chemistry
... (NOTE: the example on p. 137-8 of your textbook is the redox reaction in the Redox Titration Lab and was a question on the 2007 AP Chemistry exam.) Redox Titration- when a solution containing an oxidizing agent is titrated with a solution containing a reducing agent. The equivalence point is reache ...
... (NOTE: the example on p. 137-8 of your textbook is the redox reaction in the Redox Titration Lab and was a question on the 2007 AP Chemistry exam.) Redox Titration- when a solution containing an oxidizing agent is titrated with a solution containing a reducing agent. The equivalence point is reache ...
Curriculum Plan
... curve, Explain how solutions form and the motion of particles in solution, Define solubility and factors that affect it, Identify factors that affect rate of dissolution, Define colligative property and describe osmotic pressure, boiling point elevation, melting point depression and vapor pressure r ...
... curve, Explain how solutions form and the motion of particles in solution, Define solubility and factors that affect it, Identify factors that affect rate of dissolution, Define colligative property and describe osmotic pressure, boiling point elevation, melting point depression and vapor pressure r ...
Supplement AP Chemistry –
... 1) Write the net ionic equation for the reaction between solutions of a. nitric acid and lithium hydroxide b. ammonia and hydrogen iodide c. hydrogen fluoride and potassium cyanide d. calcium hydroxide and nitrous acid e. hydrochloric acid and a buffer (mixture of sodium sulfide and hydrosufuric aci ...
... 1) Write the net ionic equation for the reaction between solutions of a. nitric acid and lithium hydroxide b. ammonia and hydrogen iodide c. hydrogen fluoride and potassium cyanide d. calcium hydroxide and nitrous acid e. hydrochloric acid and a buffer (mixture of sodium sulfide and hydrosufuric aci ...
EXAM # 1
... NMR line-widths are related to magnetic field homogeneity, T2 relaxation time (related to molecular weight), and exchange dynamics. (d) Atomic Spectroscopy Lines are broadened by Heisenberg uncertainty principal, pressure broadening, Doppler effect and electric/magnetic fields. ...
... NMR line-widths are related to magnetic field homogeneity, T2 relaxation time (related to molecular weight), and exchange dynamics. (d) Atomic Spectroscopy Lines are broadened by Heisenberg uncertainty principal, pressure broadening, Doppler effect and electric/magnetic fields. ...
Answers - Scioly.org
... 20. The student concludes that she has synthesized ethyl butanoate. Use evidence from the two experiments to support or to refute her claim. The peak of highest mass to charge ratio is approximately 116; therefore, the unknown molecule would have a molecular mass of 116. Ethyl butanoate has the chem ...
... 20. The student concludes that she has synthesized ethyl butanoate. Use evidence from the two experiments to support or to refute her claim. The peak of highest mass to charge ratio is approximately 116; therefore, the unknown molecule would have a molecular mass of 116. Ethyl butanoate has the chem ...
Balancing Equations
... Steps to Balancing Equations There are four basic steps to balancing a chemical equation. 1. Write the correct formula for the reactants and the products. DO NOT TRY TO BALANCE IT YET! You must write the correct formulas first. ...
... Steps to Balancing Equations There are four basic steps to balancing a chemical equation. 1. Write the correct formula for the reactants and the products. DO NOT TRY TO BALANCE IT YET! You must write the correct formulas first. ...
2002 AP Chemistry Free-Response Questions
... NO CALCULATORS MAY BE USED FOR PART B. Answer Question 4 below. The Section II score weighting for this question is 15 percent. 4. Write the formulas to show the reactants and the products for any FIVE of the laboratory situations described below. Answers to more than five choices will not be graded ...
... NO CALCULATORS MAY BE USED FOR PART B. Answer Question 4 below. The Section II score weighting for this question is 15 percent. 4. Write the formulas to show the reactants and the products for any FIVE of the laboratory situations described below. Answers to more than five choices will not be graded ...
Kinetics and Equilibrium Review Page 1
... Kinetics and Equilibrium Review 35. When AgNO3(aq) is mixed with NaCl(aq), a reaction occurs which tends to go to completion and not reach equilibrium because A) a gas is formed B) water is formed C) a weak acid is formed D) a precipitate is formed 36. The vapor pressure of a liquid at a given temp ...
... Kinetics and Equilibrium Review 35. When AgNO3(aq) is mixed with NaCl(aq), a reaction occurs which tends to go to completion and not reach equilibrium because A) a gas is formed B) water is formed C) a weak acid is formed D) a precipitate is formed 36. The vapor pressure of a liquid at a given temp ...
voltammetric studies of vitamin k3 in acid aqueous solution
... vitamin K 3 in aqueous media. if the methanol contained formaldehyde as an impurity its reduction could explain the occurrence of this peak of decreasing intensity from cycle to cycle. As a matter of fact, an increase in the proportion of methanol also increased the intensity of the peak but, on the ...
... vitamin K 3 in aqueous media. if the methanol contained formaldehyde as an impurity its reduction could explain the occurrence of this peak of decreasing intensity from cycle to cycle. As a matter of fact, an increase in the proportion of methanol also increased the intensity of the peak but, on the ...
Test 9 Review - Evan`s Chemistry Corner
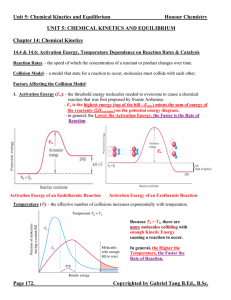
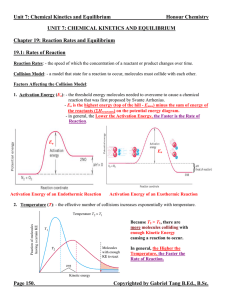
... Collision theory. In order for a reaction to occur, particles of the reactant must collide. Not all collisions cause reactions. An effective collision is one in which the colliding particles approach each other at the proper angle and with the proper amount of energy to cause a reaction. The greater ...
... Collision theory. In order for a reaction to occur, particles of the reactant must collide. Not all collisions cause reactions. An effective collision is one in which the colliding particles approach each other at the proper angle and with the proper amount of energy to cause a reaction. The greater ...
ACS Practice Test 1
... (E)place a beaker of cold water over the flame to cool the flame. 50. The electrical conductance of a solution of Ba(OH)2 slowly decreases upon the addition of H2SO4 to a minimum, and then slowly increases. The best experimental evidence for this is (A)The Ba(OH)2 solution becomes more dilute since ...
... (E)place a beaker of cold water over the flame to cool the flame. 50. The electrical conductance of a solution of Ba(OH)2 slowly decreases upon the addition of H2SO4 to a minimum, and then slowly increases. The best experimental evidence for this is (A)The Ba(OH)2 solution becomes more dilute since ...
ExamView - chemistry
... Which of the following items is NOT a compound? a. baking soda c. sucrose b. salad dressing d. table salt In the chemical reaction in which sucrose is heated and decomposes to form carbon dioxide and water, which of the following is a reactant? a. sucrose c. water b. carbon dioxide d. heat An exampl ...
... Which of the following items is NOT a compound? a. baking soda c. sucrose b. salad dressing d. table salt In the chemical reaction in which sucrose is heated and decomposes to form carbon dioxide and water, which of the following is a reactant? a. sucrose c. water b. carbon dioxide d. heat An exampl ...
rate law determination of crystal violet hydroxylation
... To find the reaction order of CV+, m, and the pseudo rate constants, k1 and k2, differential rate laws expressed in equations 3 & 4 must be integrated. (You should review integrated rate laws in your lecture text before continuing.) Integrated rate laws, when arranged in line equation form, result i ...
... To find the reaction order of CV+, m, and the pseudo rate constants, k1 and k2, differential rate laws expressed in equations 3 & 4 must be integrated. (You should review integrated rate laws in your lecture text before continuing.) Integrated rate laws, when arranged in line equation form, result i ...
20. Chemical Equilibrium
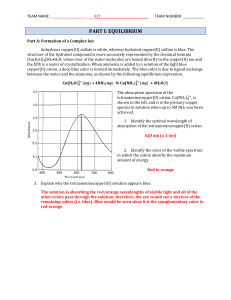
... period of time, the reaction will reach equilibrium. At this point, it may be possible to experimentally determine the concentrations of the reactants and products in the container. The concentration values are then substituted into the equilibrium expression which is then solved for Keq. Let's cons ...
... period of time, the reaction will reach equilibrium. At this point, it may be possible to experimentally determine the concentrations of the reactants and products in the container. The concentration values are then substituted into the equilibrium expression which is then solved for Keq. Let's cons ...
CHE-04 (2017)
... You can take electives (56 to 64 credits) from a minimum of TWO and a maximum of FOUR science disciplines, viz. Physics, Chemistry, Life Sciences and Mathematics. You can opt for elective courses worth a MINIMUM OF 8 CREDITS and a MAXIMUM OF 48 CREDITS from any of these four disciplines. At le ...
... You can take electives (56 to 64 credits) from a minimum of TWO and a maximum of FOUR science disciplines, viz. Physics, Chemistry, Life Sciences and Mathematics. You can opt for elective courses worth a MINIMUM OF 8 CREDITS and a MAXIMUM OF 48 CREDITS from any of these four disciplines. At le ...