Group : Nanochemical Biology Project : Tyrosine cross
... into tyrosine radicals, which then cross-react with other amino acid residues (mostly tyrosine). A major drawback of the HRP is its high reactivity, leading to dirty products that are very difficult to purify; this drawback is circumvented using a HRP mimicking DNAzyme. Furthermore, the HRP enzyme i ...
... into tyrosine radicals, which then cross-react with other amino acid residues (mostly tyrosine). A major drawback of the HRP is its high reactivity, leading to dirty products that are very difficult to purify; this drawback is circumvented using a HRP mimicking DNAzyme. Furthermore, the HRP enzyme i ...
BioN03 Amino acids, peptides, proteins Summer 2015
... With four different groups connected to the tetrahedral αcarbon atom, amino acids can be present in two forms that are mirror-images of each other (they are enantiomers). They are called L isomer and D isomer. Amino acids with their two isomers are said to be chiral (when a central carbon is bonded ...
... With four different groups connected to the tetrahedral αcarbon atom, amino acids can be present in two forms that are mirror-images of each other (they are enantiomers). They are called L isomer and D isomer. Amino acids with their two isomers are said to be chiral (when a central carbon is bonded ...
Improved amino acid analysis of feedstuffs
... of increasing pH and molarity. As no derivatization was necessary the samples were loaded directly on to the Biochrom 30 Amino Acid Analyser instrument Results: Figure 1 (below) shows an overall improvement in amino acid peak resolution using the new buffer system. Specifically, better separation is ...
... of increasing pH and molarity. As no derivatization was necessary the samples were loaded directly on to the Biochrom 30 Amino Acid Analyser instrument Results: Figure 1 (below) shows an overall improvement in amino acid peak resolution using the new buffer system. Specifically, better separation is ...
Proteins Review - kehsscience.org
... Proteins 14. Proteins in our bodies operate efficiently at about 98.6 °F (37 °C), which is normal body temperature. Study the graph below. What will happen to protein function when a person has a very high fever, say approaching 105 °F (41 °C)? ...
... Proteins 14. Proteins in our bodies operate efficiently at about 98.6 °F (37 °C), which is normal body temperature. Study the graph below. What will happen to protein function when a person has a very high fever, say approaching 105 °F (41 °C)? ...
Teaching Notes
... proteins) may have protein chains with interfaces that have hydrophobic amino acids. These proteins chains seek out and bind to partner proteins with complimentary interfaces and form functional assemblies. 5. In proteins that are composed of multiple domains, connected with flexible linker regions, ...
... proteins) may have protein chains with interfaces that have hydrophobic amino acids. These proteins chains seek out and bind to partner proteins with complimentary interfaces and form functional assemblies. 5. In proteins that are composed of multiple domains, connected with flexible linker regions, ...
amino acid
... the movement of amino acid from the extracellular space into cell. -L-amino acids are absorbed (by active transport process) more rapidly than Damino acid which absorbed slowly by simple passive diffusion. -Amino acids are absorbed by intestinal epithelial cells and released into the blood by two ty ...
... the movement of amino acid from the extracellular space into cell. -L-amino acids are absorbed (by active transport process) more rapidly than Damino acid which absorbed slowly by simple passive diffusion. -Amino acids are absorbed by intestinal epithelial cells and released into the blood by two ty ...
Study Guide for Midterm 3 – Chem 109 C
... 2. Secondary structure of a protein => Repetitive conformations of the backbone of a protein maximizing H-Bonds between the carbonyl oxygen and the H on the N in the backbone A. Alpha helix - coiling of the backbone - proline can't be in the alpha helix due to its shape causing distortion - valine, ...
... 2. Secondary structure of a protein => Repetitive conformations of the backbone of a protein maximizing H-Bonds between the carbonyl oxygen and the H on the N in the backbone A. Alpha helix - coiling of the backbone - proline can't be in the alpha helix due to its shape causing distortion - valine, ...
Talk
... Shape Representation • Graph representation of Cryo-EM volume via skeletons – 3D Skeleton [Ju 06] builds connectivity among detected helices – An edge: a detected helix or a skeleton path between two helices • Attribute: length of the helix or skeleton path ...
... Shape Representation • Graph representation of Cryo-EM volume via skeletons – 3D Skeleton [Ju 06] builds connectivity among detected helices – An edge: a detected helix or a skeleton path between two helices • Attribute: length of the helix or skeleton path ...
LESSON 2 - ASSIGNMENT 1. Differentiate between a fat and an oil
... 3. A molecule is described to you as an aliphatic, polar glycolipid. Which of the following statements must then be true for the molecule (select all that are correct); a) b) c) d) e) f) g) ...
... 3. A molecule is described to you as an aliphatic, polar glycolipid. Which of the following statements must then be true for the molecule (select all that are correct); a) b) c) d) e) f) g) ...
Supporting Information Legends Figure S1. Yeast two
... that is replaced with arginine, R). All forms of S2-SLF1 were fused with a Gal4 DNAbinding domain (BD) and a Myc-tag. Anti-Myc antibody was used to assess whether these fusion proteins were produced in yeast cells. A duplicated membrane, immunoblotted using anti-actin antibody, serves as control fo ...
... that is replaced with arginine, R). All forms of S2-SLF1 were fused with a Gal4 DNAbinding domain (BD) and a Myc-tag. Anti-Myc antibody was used to assess whether these fusion proteins were produced in yeast cells. A duplicated membrane, immunoblotted using anti-actin antibody, serves as control fo ...
PREVIEW_on_Ng_etal_STRUCTURE-MK
... arranged in a ring around a central pore aligned with a fivefold axis. The A subunit sits on top of this ring and introduces its C-terminal helical tail into the pore of the B pentamer. The authors reveal that the catalytic EcxA subunit is a metallopeptidase (MP) that is unrelated to any other AB5 f ...
... arranged in a ring around a central pore aligned with a fivefold axis. The A subunit sits on top of this ring and introduces its C-terminal helical tail into the pore of the B pentamer. The authors reveal that the catalytic EcxA subunit is a metallopeptidase (MP) that is unrelated to any other AB5 f ...
C h e m g u id e –... PROTEINS: STRUCTURE
... Some side-groups have an extra NH2 group, and some have an extra COOH group. When these come close to each other if the chain gets folded, you can get a transfer of a hydrogen ion from the COOH group to the NH2 group producing two ionic groups: COO- and NH3+. The ionic attractions between these will ...
... Some side-groups have an extra NH2 group, and some have an extra COOH group. When these come close to each other if the chain gets folded, you can get a transfer of a hydrogen ion from the COOH group to the NH2 group producing two ionic groups: COO- and NH3+. The ionic attractions between these will ...
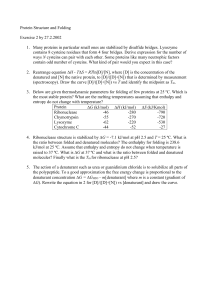
Protein Structure and Folding
... 1. Many proteins in particular small ones are stabilized by disulfide bridges. Lysozyme contains 8 cysteine residues that form 4 four bridges. Derive expression for the number of ways N cysteins can pair with each other. Some proteins like many neutrophic factors contain odd number of cysteins. What ...
... 1. Many proteins in particular small ones are stabilized by disulfide bridges. Lysozyme contains 8 cysteine residues that form 4 four bridges. Derive expression for the number of ways N cysteins can pair with each other. Some proteins like many neutrophic factors contain odd number of cysteins. What ...
Proteins are biopolymers construced from similar building blocks
... Proteins are biopolymers construced from similar building blocks called amino acids. The unique feature is that these polypeptide chains are folded in a certain three-dimensional structure (called native structure), which enables them to perform their biological funtion. Studies on protein structure ...
... Proteins are biopolymers construced from similar building blocks called amino acids. The unique feature is that these polypeptide chains are folded in a certain three-dimensional structure (called native structure), which enables them to perform their biological funtion. Studies on protein structure ...
Health and Wellness
... Journal Entry 2 • “Nothing in excess, moderation is best in all things.” • What does this quote mean to you? – How does this quote pertain to one’s diet, lifestyle, and level of physical activity? *All journal entries must be at least ½ page in length to obtain full credit. ...
... Journal Entry 2 • “Nothing in excess, moderation is best in all things.” • What does this quote mean to you? – How does this quote pertain to one’s diet, lifestyle, and level of physical activity? *All journal entries must be at least ½ page in length to obtain full credit. ...
The Chemistry of Life: *Inorganic compounds– compounds that lack
... and include things such as water, and trace elements like sodium, magnesium, iron, calcium, etc. *Organic Compounds – compounds containing the element carbon. There are four major classes of organic compounds listed below. These compounds are the cornerstone of all living things on this planet. 1. C ...
... and include things such as water, and trace elements like sodium, magnesium, iron, calcium, etc. *Organic Compounds – compounds containing the element carbon. There are four major classes of organic compounds listed below. These compounds are the cornerstone of all living things on this planet. 1. C ...
Protein Architecture and Structure Alignment
... “The three-dimensional structure of a native protein in its normal physiological milieu (solvent, pH, ionic strength, presence of other components such as metal ions or prosthetic groups, temperature, etc.) is the one in which the Gibbs free energy of the whole system is lowest; that is, that the na ...
... “The three-dimensional structure of a native protein in its normal physiological milieu (solvent, pH, ionic strength, presence of other components such as metal ions or prosthetic groups, temperature, etc.) is the one in which the Gibbs free energy of the whole system is lowest; that is, that the na ...
Proteins * Structure and Function
... such that one end of the molecule has a positive electrical charge and the other side has a negative charge. If this is the case, the molecule is called a polar molecule, meaning that it has electrical poles. Otherwise, it is called a non-polar molecule. • Whether molecules are polar or non-polar de ...
... such that one end of the molecule has a positive electrical charge and the other side has a negative charge. If this is the case, the molecule is called a polar molecule, meaning that it has electrical poles. Otherwise, it is called a non-polar molecule. • Whether molecules are polar or non-polar de ...
All About Proteins Proteins are highly folded polymers constructed
... Proteins are highly folded polymers constructed from monomers called amino acids. There are 20 Amino Acids and ALL have the same structure except for the R group or side chain. Notice the variety in the R groups shown below – and no, you do NOT need to memorize them. You DO need to recognize and be ...
... Proteins are highly folded polymers constructed from monomers called amino acids. There are 20 Amino Acids and ALL have the same structure except for the R group or side chain. Notice the variety in the R groups shown below – and no, you do NOT need to memorize them. You DO need to recognize and be ...
Answer Sheet (LEGO Lab)
... 5. Which is likely to have a GREATER effect on enzyme activity? a) Changing a hydrophobic amino acid to a hydrophilic amino acid OR b) Changing a hydrophobic amino acid to another hydrophobic amino acid? Explain. ...
... 5. Which is likely to have a GREATER effect on enzyme activity? a) Changing a hydrophobic amino acid to a hydrophilic amino acid OR b) Changing a hydrophobic amino acid to another hydrophobic amino acid? Explain. ...
Chapter 5 Separations: I) Based on Charge or pI A) Electrophoresis
... Carboxypeptidases will cleave amino acids sequentially from the C-terminus. There are different types (A, B, C, and Y) that are effective for different amino acids. Aminopeptidases will cleave amino acids sequentially from the N-terminus. Determination of 3-D structure of proteins X-ray diffraction ...
... Carboxypeptidases will cleave amino acids sequentially from the C-terminus. There are different types (A, B, C, and Y) that are effective for different amino acids. Aminopeptidases will cleave amino acids sequentially from the N-terminus. Determination of 3-D structure of proteins X-ray diffraction ...
Proteins - Fort Thomas Independent Schools
... “water loving” amino acids try to stay in water in cell ...
... “water loving” amino acids try to stay in water in cell ...