Naming Alkenes - Organic Chemistry Help!
... - hybrid orbitals explain the sigma bond behavior well - molecular orbitals will be used to discuss pi bonds - like hybrid theory, atomic orbitals are mixed to form new orbitals (in this case, the new orbitals are molecular orbitals) - In this case, orbitals from different atoms are mixed - The numb ...
... - hybrid orbitals explain the sigma bond behavior well - molecular orbitals will be used to discuss pi bonds - like hybrid theory, atomic orbitals are mixed to form new orbitals (in this case, the new orbitals are molecular orbitals) - In this case, orbitals from different atoms are mixed - The numb ...
6.1 Coulomb interaction energy among charged particles in an atom
... 6.2 Allowed energy levels of the electron in H-atom The electronic Hamiltonian in atomic units for the electron in H-atom (Z=1) is eq 6.19 where r is the distance between the electron and the nucleus, in bohrs. The fact that the potential energy depends only on r suggests that it should be easier to ...
... 6.2 Allowed energy levels of the electron in H-atom The electronic Hamiltonian in atomic units for the electron in H-atom (Z=1) is eq 6.19 where r is the distance between the electron and the nucleus, in bohrs. The fact that the potential energy depends only on r suggests that it should be easier to ...
2. Atomic Structure 2.1 Historical Development of Atomic Theory
... “The more precisely the position is determined, the less precisely the momentum is known in this instant, and vice versa.” (Heisenberg, 1927) ...
... “The more precisely the position is determined, the less precisely the momentum is known in this instant, and vice versa.” (Heisenberg, 1927) ...
Lecture 4: methods and terminology, part II
... integrals are the most expensive part of the Hartree-Fock theory calculations, and the semi-empirical methods attempt to replace them with empirical parameters. Approximations Core electrons are not considered or approximated by effective core potentials. Minimal basis set (one function per orbital) ...
... integrals are the most expensive part of the Hartree-Fock theory calculations, and the semi-empirical methods attempt to replace them with empirical parameters. Approximations Core electrons are not considered or approximated by effective core potentials. Minimal basis set (one function per orbital) ...
Final Review
... many-electron atom have different energies? The actual wavefunction of a manyelectron atom is a very complicated function of the coordinates of all of the electrons. In the orbital approximation, we suppose that a reasonable first approx. to the exact wavefunction is obtained by taking the product o ...
... many-electron atom have different energies? The actual wavefunction of a manyelectron atom is a very complicated function of the coordinates of all of the electrons. In the orbital approximation, we suppose that a reasonable first approx. to the exact wavefunction is obtained by taking the product o ...
Atomic_Orbitals
... electron absorbs a quantum of energy, it moves up to a higher orbital. When the electron falls from a high orbital to a lower orbital, energy is released, and we see light. Wintergreen mint is an example We will also see this in our spectroscopy and flame test labs! ...
... electron absorbs a quantum of energy, it moves up to a higher orbital. When the electron falls from a high orbital to a lower orbital, energy is released, and we see light. Wintergreen mint is an example We will also see this in our spectroscopy and flame test labs! ...
Hybridization of atomic orbitals In general VSEPR predicts the
... Hybridization of atomic orbitals In general VSEPR predicts the shape of molecules and ions accurately CH4 : tetrahedral Four equal bonds with equal HCH angles A covalent bond is formed by sharing two electrons by two atoms Imagine an orbital (containing 1 electron) from one atom overlaps with an orb ...
... Hybridization of atomic orbitals In general VSEPR predicts the shape of molecules and ions accurately CH4 : tetrahedral Four equal bonds with equal HCH angles A covalent bond is formed by sharing two electrons by two atoms Imagine an orbital (containing 1 electron) from one atom overlaps with an orb ...
Hybridization of atomic orbitals In general VSEPR predicts the
... Hybridization of atomic orbitals In general VSEPR predicts the shape of molecules and ions accurately CH4 : tetrahedral Four equal bonds with equal HCH angles A covalent bond is formed by sharing two electrons by two atoms Imagine an orbital (containing 1 electron) from one atom overlaps with an orb ...
... Hybridization of atomic orbitals In general VSEPR predicts the shape of molecules and ions accurately CH4 : tetrahedral Four equal bonds with equal HCH angles A covalent bond is formed by sharing two electrons by two atoms Imagine an orbital (containing 1 electron) from one atom overlaps with an orb ...
Atomic Bonding - New Academic Science
... accurately to determine the position of the electron, but it is possible to calculate the probability of finding the electron at any point around the nucleus. Within a hydrogen atom the probability of distribution of electrons is spherical around the nucleus and it is possible to draw a spherical bo ...
... accurately to determine the position of the electron, but it is possible to calculate the probability of finding the electron at any point around the nucleus. Within a hydrogen atom the probability of distribution of electrons is spherical around the nucleus and it is possible to draw a spherical bo ...
Orbital Hybridisation www.AssignmentPoint.com In chemistry
... chemical bonds in valence bond theory. Hybrid orbitals are very useful in the explanation of molecular geometry and atomic bonding properties. Although sometimes taught together with the valence shell electron-pair repulsion (VSEPR) theory, valence bond and hybridisation are in fact not related to t ...
... chemical bonds in valence bond theory. Hybrid orbitals are very useful in the explanation of molecular geometry and atomic bonding properties. Although sometimes taught together with the valence shell electron-pair repulsion (VSEPR) theory, valence bond and hybridisation are in fact not related to t ...
Electron Configuration
... F sublevels The f sublevel is composed of 7 f orbitals. Each orbital is each in the amount of energy. A total of 14 electrons can be found in an f sublevel. ...
... F sublevels The f sublevel is composed of 7 f orbitals. Each orbital is each in the amount of energy. A total of 14 electrons can be found in an f sublevel. ...
do with electron orbitals?
... What are the boundary conditions on the wavefunction () in r ? a. must go to 0 at r=0 b. must go to 0 at r=infinity c. at infinity must equal at 0 d. A and B e. A, B, and C must be normalizable, so needs to go to zero … Also physically makes sense … not probable to find electron there ...
... What are the boundary conditions on the wavefunction () in r ? a. must go to 0 at r=0 b. must go to 0 at r=infinity c. at infinity must equal at 0 d. A and B e. A, B, and C must be normalizable, so needs to go to zero … Also physically makes sense … not probable to find electron there ...
Introduction to Computational Chemistry
... biomolecular simulations (AG Wennmohs). The intention of these experiments is to provide a feeling of how one could tackle problems of actual chemical relevance with computational chemistry. In order to succ ...
... biomolecular simulations (AG Wennmohs). The intention of these experiments is to provide a feeling of how one could tackle problems of actual chemical relevance with computational chemistry. In order to succ ...
Molecular Quadratic Response Properties with Inclusion of Relativity Johan Henriksson
... the light of these developments, Paul Dirac stated: 14 “The underlying physical laws necessary for the mathematical theory of a large part of physics and the whole of chemistry are thus completely known, and the difficulty is only that the exact application of these laws leads to equations much too ...
... the light of these developments, Paul Dirac stated: 14 “The underlying physical laws necessary for the mathematical theory of a large part of physics and the whole of chemistry are thus completely known, and the difficulty is only that the exact application of these laws leads to equations much too ...
Transition metal configurations and limitations of the orbital
... The Schrodinser eauation mav only be solved exactlv for the case of the two-particle system in the hydrogen atom. As soon as we turn to the three-body problem, we must introduce approximations in order to proceed. In the crudest of all approximations, all interactions between electrons are completel ...
... The Schrodinser eauation mav only be solved exactlv for the case of the two-particle system in the hydrogen atom. As soon as we turn to the three-body problem, we must introduce approximations in order to proceed. In the crudest of all approximations, all interactions between electrons are completel ...
I Complex Ion Formation
... when the labels of electrons one and two are interchanged and hence the electrons appear distinguishable. However, the linear combinations (sums) $,(1)9J2(2) $1(2)$2(l) and h(l)$2(2) - W W ( 1 ) conserve the indistinguishability since the values of their squares are unchanged when the labels are int ...
... when the labels of electrons one and two are interchanged and hence the electrons appear distinguishable. However, the linear combinations (sums) $,(1)9J2(2) $1(2)$2(l) and h(l)$2(2) - W W ( 1 ) conserve the indistinguishability since the values of their squares are unchanged when the labels are int ...
1.1 Construction of two band models
... We first illustrate the form of our two band models and how to construct it from a simple square lattice with , and orbitals. The two band model takes the following form ...
... We first illustrate the form of our two band models and how to construct it from a simple square lattice with , and orbitals. The two band model takes the following form ...
Quantum States of the- Trapped Electron for an Interstitial Ion*
... In the present calculation, the crystal is assumed to bc a dielectric medium and the net point charge for the interstitial ion is positive and unity. When the electron under discussion is moving in the region very close to the intcrstitial ion, its electric field is almost completely shielded by the ...
... In the present calculation, the crystal is assumed to bc a dielectric medium and the net point charge for the interstitial ion is positive and unity. When the electron under discussion is moving in the region very close to the intcrstitial ion, its electric field is almost completely shielded by the ...
Quantum Mechanics
... Are places in an atom where there is a high likelihood of finding an electron. (Heisenberg Priniple) No more than two electrons can be in any orbital. The is called the Pauli Exclusion Principle. There are three possibilities for electrons in an orbital. The orbitals have 0,1, or 2 electons containe ...
... Are places in an atom where there is a high likelihood of finding an electron. (Heisenberg Priniple) No more than two electrons can be in any orbital. The is called the Pauli Exclusion Principle. There are three possibilities for electrons in an orbital. The orbitals have 0,1, or 2 electons containe ...
Many-body approaches to studies of electronic systems: Hartree-Fock theory and Density
... wave function. All results are in atomic units, meaning that the energy is given by enl = −Z 2 /2n2 and the radius is dimensionless. We obtain then a modified single-particle eigenfunction which in turn can be used as an input in a variational Monte Carlo calculation of the ground state of a specifi ...
... wave function. All results are in atomic units, meaning that the energy is given by enl = −Z 2 /2n2 and the radius is dimensionless. We obtain then a modified single-particle eigenfunction which in turn can be used as an input in a variational Monte Carlo calculation of the ground state of a specifi ...
Atomic Orbitals Lab - North Carolina High School Computational
... equation and the Hamiltonian are quite complicated): 1. The kinetic energy (KE) of the electrons 2. The repulsive force between electrons 3. The repulsive force between nuclei (for molecules) 4. The attractive force between electrons and nuclei The value “Ψ” represents the wavefunction, a mathematic ...
... equation and the Hamiltonian are quite complicated): 1. The kinetic energy (KE) of the electrons 2. The repulsive force between electrons 3. The repulsive force between nuclei (for molecules) 4. The attractive force between electrons and nuclei The value “Ψ” represents the wavefunction, a mathematic ...
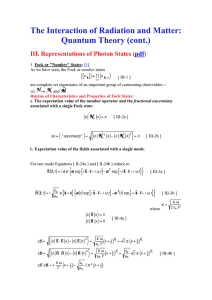
The Interaction of Radiation and Matter: Quantum
... The phase operators defined by Equation [ III-36 ] do have the felicitous or classically analogous property of revealing magnitude independent information, but unfortunately they are nonHermitian operators -- i.e. -- and, hence, cannot represent observables. However, they may be paired into operator ...
... The phase operators defined by Equation [ III-36 ] do have the felicitous or classically analogous property of revealing magnitude independent information, but unfortunately they are nonHermitian operators -- i.e. -- and, hence, cannot represent observables. However, they may be paired into operator ...
BINDING ENERGIES OF EXCITONS IN QUANTUM WELL
... Excitons in dimensional reduced structures have been intensively studied by various theoretical methods. In most approaches variational procedures have been included and a separation ansatz for the electron and hole wave functions has been used [1, 2]. The dependence of the excitor binding energy on ...
... Excitons in dimensional reduced structures have been intensively studied by various theoretical methods. In most approaches variational procedures have been included and a separation ansatz for the electron and hole wave functions has been used [1, 2]. The dependence of the excitor binding energy on ...
Quantum Numbers and Electronic Configuration
... generally follows this principle. According to this principle, electrons enter into states in order of the states increasing energy. Electrons are reluctant to pair up with another in the same atomic orbital (due to repulsions) and to go to a higher energy level than is necessary. When faced with th ...
... generally follows this principle. According to this principle, electrons enter into states in order of the states increasing energy. Electrons are reluctant to pair up with another in the same atomic orbital (due to repulsions) and to go to a higher energy level than is necessary. When faced with th ...
Electrons in Atoms
... But is there a theory that could be used to predict the behaviour of the electron in the H-atom and hence predict its spectrum? The answer, of course, is yes; Quantum Mechanics and the Schrödinger equation. The possible energies of an electron in an H-atom (energy levels) and its associated ‘s ...
... But is there a theory that could be used to predict the behaviour of the electron in the H-atom and hence predict its spectrum? The answer, of course, is yes; Quantum Mechanics and the Schrödinger equation. The possible energies of an electron in an H-atom (energy levels) and its associated ‘s ...