4.7 The two atoms containing molecule
... to the Coulomb interaction between the electrons are not understood until now, but they are most probably the reason for the unexpected high transition temperatures in these superconductors. To get a feeling for these problems and the strong coupling between magnetic properties and the Coulomb inter ...
... to the Coulomb interaction between the electrons are not understood until now, but they are most probably the reason for the unexpected high transition temperatures in these superconductors. To get a feeling for these problems and the strong coupling between magnetic properties and the Coulomb inter ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... Discuss the Pauli Exclusion Principle in quantum mechanics applied to He atom in its ground state. ...
... Discuss the Pauli Exclusion Principle in quantum mechanics applied to He atom in its ground state. ...
Solutions of the Schrödinger equation for the ground helium by finite
... Multi-body Coulomb problems are traditional challenging problems [1]. The failure of theory to describe precisely the system stimulated many mathematicians and physicists to devote themselves in using various methods to obtain the energies and other expectation values. Few-electron systems like heli ...
... Multi-body Coulomb problems are traditional challenging problems [1]. The failure of theory to describe precisely the system stimulated many mathematicians and physicists to devote themselves in using various methods to obtain the energies and other expectation values. Few-electron systems like heli ...
Quantum Mechanical Model of the Atom
... MODEL OF THE ATOM ESSENTIAL QUESTION: WHAT IS THE CURRENT MODEL OF THE ATOM? ...
... MODEL OF THE ATOM ESSENTIAL QUESTION: WHAT IS THE CURRENT MODEL OF THE ATOM? ...
Riemannian method in quantum field theory about curved space-time
... The essential content of my talk is now in the following statement: One can reasonably associate to every V~ a Hilbert space F s of one-particle states which are realized by measurable function concentrated on V~. There is further defined a positive semidefinite operator H s acting on this Hilbert s ...
... The essential content of my talk is now in the following statement: One can reasonably associate to every V~ a Hilbert space F s of one-particle states which are realized by measurable function concentrated on V~. There is further defined a positive semidefinite operator H s acting on this Hilbert s ...
Chemistry 2000 Review: quantum mechanics of
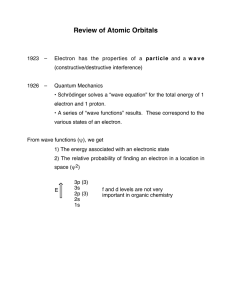
... This equation was know to belong to a special class known as an eigenvector equation: an operator acts on a function (ψ) and generates a scalar times the same function Ψ is known as the wavefunction of the electron: there are an infinite number of such wavefunctions, each of which is characterized b ...
... This equation was know to belong to a special class known as an eigenvector equation: an operator acts on a function (ψ) and generates a scalar times the same function Ψ is known as the wavefunction of the electron: there are an infinite number of such wavefunctions, each of which is characterized b ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... Explain each of the integrals in the above equation and their significance. 16. The spacing between adjacent rotational lines in the spectrum of HCl molecule is 6.33 x 1011s-1. Calculate the moment of inertia of HCl molecule and the internuclear spacing if the atomic masses are H = 1.008 and Cl = 34 ...
... Explain each of the integrals in the above equation and their significance. 16. The spacing between adjacent rotational lines in the spectrum of HCl molecule is 6.33 x 1011s-1. Calculate the moment of inertia of HCl molecule and the internuclear spacing if the atomic masses are H = 1.008 and Cl = 34 ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI-600034 M.Sc. Part-A NOVEMBER 2015
... Convert the Cartesian coordinate (-1,1,-√2) into spherical polar coordinates. Derive time independent Schrodinger wave equation from time dependent equation. The force constant for H79Br is 392 Nm-1. Calculate the fundamental vibrational frequency and zero point energy of H79Br. Use the method of se ...
... Convert the Cartesian coordinate (-1,1,-√2) into spherical polar coordinates. Derive time independent Schrodinger wave equation from time dependent equation. The force constant for H79Br is 392 Nm-1. Calculate the fundamental vibrational frequency and zero point energy of H79Br. Use the method of se ...
Pdf
... The Hartree–Fock self-consistent-field approximation has provided an invaluable conceptual framework and a standard computational procedure for atomic and molecular quantum theory. Its shortcomings are significant however, and require remediation. Mo” ller–Plesset perturbation theory offers a popula ...
... The Hartree–Fock self-consistent-field approximation has provided an invaluable conceptual framework and a standard computational procedure for atomic and molecular quantum theory. Its shortcomings are significant however, and require remediation. Mo” ller–Plesset perturbation theory offers a popula ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 23. a) Explain the use of Born-Oppenheimer approximation with a suitable example. b) Derive the time-independent Schroedinger equation from the time-dependent and prove that the property such as electron density is time independent although the wave function describing an electron is time dependent. ...
... 23. a) Explain the use of Born-Oppenheimer approximation with a suitable example. b) Derive the time-independent Schroedinger equation from the time-dependent and prove that the property such as electron density is time independent although the wave function describing an electron is time dependent. ...
collective states of 2d electron-hole system under the influence of
... magnetoexciton ground state energy, and the energy of the single-particle elementary excitations were obtained. The energy per one e–h pair inside the electron-hole droplets found to be situated on the energy scale lower than the value of the chemical potential of the Bose–Einstein condensed magneto ...
... magnetoexciton ground state energy, and the energy of the single-particle elementary excitations were obtained. The energy per one e–h pair inside the electron-hole droplets found to be situated on the energy scale lower than the value of the chemical potential of the Bose–Einstein condensed magneto ...
Chapter 2 Molecular Mechanics
... separated because the nuclei move very slowly with respect to the electrons. • The Born-Oppenheimer (BO) approximation allows the two parts of the problem can be solved independently. – The Electronic Hamiltonian neglecting the kinetic energy term for the nuclei. ...
... separated because the nuclei move very slowly with respect to the electrons. • The Born-Oppenheimer (BO) approximation allows the two parts of the problem can be solved independently. – The Electronic Hamiltonian neglecting the kinetic energy term for the nuclei. ...
Lecture 5
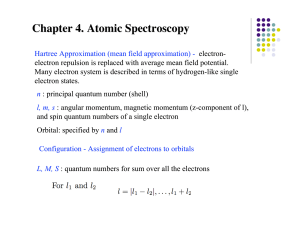
... Hartree Approximation (mean field approximation) - electronelectron repulsion is replaced with average mean field potential. Many electron system is described in terms of hydrogen-like single electron states. n : principal quantum number (shell) l, m, s : angular momentum, magnetic momentum (z-compo ...
... Hartree Approximation (mean field approximation) - electronelectron repulsion is replaced with average mean field potential. Many electron system is described in terms of hydrogen-like single electron states. n : principal quantum number (shell) l, m, s : angular momentum, magnetic momentum (z-compo ...
Exercise 6
... (Saint Petersburg State University), and he taught there from 1924, becoming a professor in 1932. The Hartree-Fock equation, improved by him in 1930, became a basic approximation method for calculations involving multielectron atoms in quantum chemistry. He also introduced the Fock representation (1 ...
... (Saint Petersburg State University), and he taught there from 1924, becoming a professor in 1932. The Hartree-Fock equation, improved by him in 1930, became a basic approximation method for calculations involving multielectron atoms in quantum chemistry. He also introduced the Fock representation (1 ...