Notes Unit 5-4
... • 1 mole = 6.02 x 1023 particles • Mole = amount of a substance “mol” • Avogadro’s Number • Based on the weight of carbon-12 atoms. ...
... • 1 mole = 6.02 x 1023 particles • Mole = amount of a substance “mol” • Avogadro’s Number • Based on the weight of carbon-12 atoms. ...
Name - Holland Public Schools
... c. ∆ HE positive; Products are HOT! III. Let’s Get It Started! A. Activation Energy (AE) 1. Energy needed to get a reaction started 2. Every reaction has some AE ...
... c. ∆ HE positive; Products are HOT! III. Let’s Get It Started! A. Activation Energy (AE) 1. Energy needed to get a reaction started 2. Every reaction has some AE ...
Packet #6- Ionic and Covalent Bonding
... Ions are electrically charged particles formed when atoms lose or gain electrons. This loss or gain leaves a complete highest energy level, so the electronic structure of an ion is the same as that of a noble gas - such as a helium, neon or argon. Metal atoms and non-metal atoms go in opposite direc ...
... Ions are electrically charged particles formed when atoms lose or gain electrons. This loss or gain leaves a complete highest energy level, so the electronic structure of an ion is the same as that of a noble gas - such as a helium, neon or argon. Metal atoms and non-metal atoms go in opposite direc ...
Preview to Mole Activity #2 preview_to_mole_activity_21
... 8) A bottle contains a 32 gram sample of sulfur. How many atoms do you think are in this bottle? A long time ago chemists discovered what you just discovered by answering question 8. If they were talking about the mass of one atom of an element they talked about its mass in amu’s. This was not very ...
... 8) A bottle contains a 32 gram sample of sulfur. How many atoms do you think are in this bottle? A long time ago chemists discovered what you just discovered by answering question 8. If they were talking about the mass of one atom of an element they talked about its mass in amu’s. This was not very ...
Scientific Measurement
... of the principal energy level and the distance to the atom’s nucleus. _____26. I can identify an electron configuration that shows an atom in the excited state. ...
... of the principal energy level and the distance to the atom’s nucleus. _____26. I can identify an electron configuration that shows an atom in the excited state. ...
17588_lecture10-11_11795_laser-and-its-applications2
... in an excited state E2, it stimulates the atom to drop or decay to the lower state E1. In this process, the atom releases a photon of the same energy, direction, phase and polarization as that of the photon passing by, the net effect is two identical photons (2h) in the place of one, or an increase ...
... in an excited state E2, it stimulates the atom to drop or decay to the lower state E1. In this process, the atom releases a photon of the same energy, direction, phase and polarization as that of the photon passing by, the net effect is two identical photons (2h) in the place of one, or an increase ...
7-1 Avogadro`s Number and Molar Conversions Objectives: • Identify
... 1. Most Elements Are Mixtures of Isotopes. 2. The periodic table reports the average atomic mass in amu. 3. To find a monatomic element’s molar mass, use the average atomic mass, but instead of having units of amu, the molar mass will have units of g/mol. B Chemical Formulas and Moles 1. Formulas Ex ...
... 1. Most Elements Are Mixtures of Isotopes. 2. The periodic table reports the average atomic mass in amu. 3. To find a monatomic element’s molar mass, use the average atomic mass, but instead of having units of amu, the molar mass will have units of g/mol. B Chemical Formulas and Moles 1. Formulas Ex ...
End Show
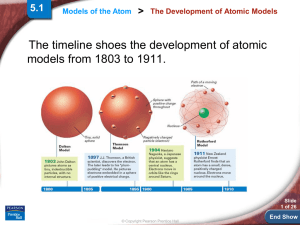
... explain the chemical properties of elements. Rutherford’s atomic model could not explain why objects change color when heated. ...
... explain the chemical properties of elements. Rutherford’s atomic model could not explain why objects change color when heated. ...
What is remote sensing?
... Making of two measurements rather then one in the atm window region (near 11 m ) called split window. The two channel see the same absorbers but in different amount. The aim of the split window is to correct atm attenuation (mostly due to water vapour) to estimate better surface temperature. The ...
... Making of two measurements rather then one in the atm window region (near 11 m ) called split window. The two channel see the same absorbers but in different amount. The aim of the split window is to correct atm attenuation (mostly due to water vapour) to estimate better surface temperature. The ...
Outline Chapter 10 The Periodic Law
... Ionic bond = formed when electrons are transferred between two or more atoms and the resulting ions of opposite charge attract each other. 10-14. Ionic Compounds When a metal atom combines with a nonmetal atom to form an ionic compound, the chemical formula of the ionic compound formed can be determ ...
... Ionic bond = formed when electrons are transferred between two or more atoms and the resulting ions of opposite charge attract each other. 10-14. Ionic Compounds When a metal atom combines with a nonmetal atom to form an ionic compound, the chemical formula of the ionic compound formed can be determ ...
Absorption of Radiation
... wavelength of plane polarized light, concentration & number of symmetric molecules •circularly polarized light: the electric field vector is rotating around the axis of light propagation. • electric field vector can rotate in either the right or left direction, and the light is called right (Clockwi ...
... wavelength of plane polarized light, concentration & number of symmetric molecules •circularly polarized light: the electric field vector is rotating around the axis of light propagation. • electric field vector can rotate in either the right or left direction, and the light is called right (Clockwi ...
Worksheet
... 37. Which of the following is a correct interpretation of the results of Rutherford's experiments in which gold atoms were bombarded with alpha particles? (A) Atoms have equal numbers of positive and negative charges. (B) Electrons in atoms are arranged in shells. (C) Neutrons are at the center of a ...
... 37. Which of the following is a correct interpretation of the results of Rutherford's experiments in which gold atoms were bombarded with alpha particles? (A) Atoms have equal numbers of positive and negative charges. (B) Electrons in atoms are arranged in shells. (C) Neutrons are at the center of a ...
Mass Spectrometry (MS) Primer
... Having two analysers increases the selectivity. The ion signal is reduced during the transmission, but the chemical noise, which is a major limitation for complex samples, is also largely decreased, leading to an improvement of the signal to noise ratio. It is thus possible to do quantitative analys ...
... Having two analysers increases the selectivity. The ion signal is reduced during the transmission, but the chemical noise, which is a major limitation for complex samples, is also largely decreased, leading to an improvement of the signal to noise ratio. It is thus possible to do quantitative analys ...
1 - PLK Vicwood KT Chong Sixth Form College
... (c) An a.c. is fed to a flat, circular coil as shown. The magnetic field produced is detected by an axial search coil held vertically at the centre of the coil. The location/plane of the search coil should be kept fixed relative to the coil. A CRO indicates the induced e.m.f. picked up by the search ...
... (c) An a.c. is fed to a flat, circular coil as shown. The magnetic field produced is detected by an axial search coil held vertically at the centre of the coil. The location/plane of the search coil should be kept fixed relative to the coil. A CRO indicates the induced e.m.f. picked up by the search ...
Investigating Chemistry - Chemistry at Winthrop University
... Groups 13-16 are referred to by the first element or simply the group number. Group 17 is the Halogens. Group 18 is the Noble Gases. Elements 58-71 and 90-103 are called the Inner Transition Metals. ...
... Groups 13-16 are referred to by the first element or simply the group number. Group 17 is the Halogens. Group 18 is the Noble Gases. Elements 58-71 and 90-103 are called the Inner Transition Metals. ...
Bose-Einstein Condensation in Cold Atoms: a New State of Matter
... unlike photons, cannot be created or destroyed, so amplification of an atom laser (see figure 4)involves pumping more atoms into the ground quantum state through quantum coherence. Matter waves are also affected by gravity, as their masses are large compared to photons (that have zero mass).[9] Appl ...
... unlike photons, cannot be created or destroyed, so amplification of an atom laser (see figure 4)involves pumping more atoms into the ground quantum state through quantum coherence. Matter waves are also affected by gravity, as their masses are large compared to photons (that have zero mass).[9] Appl ...
The collision theory of reactions
... at 300 K only 1 in 1011 collisions between H2 and N2 results in a reaction! Even at 800 K only 1 in 104 collisions results in a reaction. The collision theory says: Reactions occur when molecules collide with a certain minimum kinetic energy. The more frequent these collisions, the faster the rate o ...
... at 300 K only 1 in 1011 collisions between H2 and N2 results in a reaction! Even at 800 K only 1 in 104 collisions results in a reaction. The collision theory says: Reactions occur when molecules collide with a certain minimum kinetic energy. The more frequent these collisions, the faster the rate o ...
PDF: 6 pages, 57 KB - Quantum aspects of the world
... new value of y. We can continue in this way to determine the how the function y changes along a set of successive positions. To carry out the stepwise solution of the Schrödinger equation, we need a potential energy function, the values of the wavefunction at the first two mesh points, and value of ...
... new value of y. We can continue in this way to determine the how the function y changes along a set of successive positions. To carry out the stepwise solution of the Schrödinger equation, we need a potential energy function, the values of the wavefunction at the first two mesh points, and value of ...
Adv review key
... B) Valence electrons- outer shell electrons C) Metals a. Lend valence electrons b. 1 – 4 valence electrons c. Form positive ions ( more protons than electrons) D) Nonmetals a. Borrow valence electrons b. 4 - 8 valence electrons c. Form negative ions (more electrons than protons) E) Metals lend and n ...
... B) Valence electrons- outer shell electrons C) Metals a. Lend valence electrons b. 1 – 4 valence electrons c. Form positive ions ( more protons than electrons) D) Nonmetals a. Borrow valence electrons b. 4 - 8 valence electrons c. Form negative ions (more electrons than protons) E) Metals lend and n ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.