here.
... the cone with x-component of angular momentum equal to ~m x as −~m x . So by symmetry we would expect the expectation value of L x in the state Ylm to vanish, as it does. It is important to realize that this cone does not tell us where the particle is likely to be found, it only gives some crude in ...
... the cone with x-component of angular momentum equal to ~m x as −~m x . So by symmetry we would expect the expectation value of L x in the state Ylm to vanish, as it does. It is important to realize that this cone does not tell us where the particle is likely to be found, it only gives some crude in ...
CHEM 101 Final (Term 141)
... 23. The vapor pressure of a liquid increases four times when the temperature is raised from 50.0 C to 100. C. Calculate the molar heat of vaporization. A) B) C) D) E) ...
... 23. The vapor pressure of a liquid increases four times when the temperature is raised from 50.0 C to 100. C. Calculate the molar heat of vaporization. A) B) C) D) E) ...
Chem 11 Notes Booklet (pdf version)
... ◘ The modern periodic table arranges the elements in order of increasing atomic number. ◘ Metals are separated from nonmetals by the “staircase line”. metals - shiny, malleable, ductile, conductors of heat and electricity. ◘ The columns are families (groups) of elements having similar chemical prope ...
... ◘ The modern periodic table arranges the elements in order of increasing atomic number. ◘ Metals are separated from nonmetals by the “staircase line”. metals - shiny, malleable, ductile, conductors of heat and electricity. ◘ The columns are families (groups) of elements having similar chemical prope ...
Packet #7- Chemical Reactions
... In sodium oxide, there are two sodium atoms for every oxygen atom, so we show its formula as Na2O. The small 2 after an element tells you there are two atoms of that particular element in each molecule. For example, the water molecule H2O has two hydrogen atoms. Notice that the 2 is written as a sub ...
... In sodium oxide, there are two sodium atoms for every oxygen atom, so we show its formula as Na2O. The small 2 after an element tells you there are two atoms of that particular element in each molecule. For example, the water molecule H2O has two hydrogen atoms. Notice that the 2 is written as a sub ...
Camp 1 - drjosephryan.com Home Page
... But while it tells us what the reactants and products are and the physical state of each, it is incomplete because it is not balanced ...
... But while it tells us what the reactants and products are and the physical state of each, it is incomplete because it is not balanced ...
Answers - Scioly.org
... 20. The student concludes that she has synthesized ethyl butanoate. Use evidence from the two experiments to support or to refute her claim. The peak of highest mass to charge ratio is approximately 116; therefore, the unknown molecule would have a molecular mass of 116. Ethyl butanoate has the chem ...
... 20. The student concludes that she has synthesized ethyl butanoate. Use evidence from the two experiments to support or to refute her claim. The peak of highest mass to charge ratio is approximately 116; therefore, the unknown molecule would have a molecular mass of 116. Ethyl butanoate has the chem ...
Electrochemistry
... and charges. (Look at medium) Step 4: Add the two half-reactions together and balance the final equation by inspection. The electrons on both sides must cancel. (Be sure they are equal) Step 5: Verify that the equation contains the same type and numbers of atoms and the same charges on both sides of ...
... and charges. (Look at medium) Step 4: Add the two half-reactions together and balance the final equation by inspection. The electrons on both sides must cancel. (Be sure they are equal) Step 5: Verify that the equation contains the same type and numbers of atoms and the same charges on both sides of ...
Chemistry – Higher level Marking Scheme
... having different mass numbers (different numbers of neutrons) ...
... having different mass numbers (different numbers of neutrons) ...
Introductory Review
... sugar completely dissolved in water. Heterogeneous mixture: A mixture in which the individual components remain physically separate and can be seen as separate components. Example: a mixture of sugar and sand. ...
... sugar completely dissolved in water. Heterogeneous mixture: A mixture in which the individual components remain physically separate and can be seen as separate components. Example: a mixture of sugar and sand. ...
Optical properties of the human tissue
... with integrating spheres. This method allows one to determine the absorption and the reduced scattering coefficients of a turbid media from the measured values of the total transmittance and the diffuse reflectance. In these calculations the anisotropy factor can be fixed as 0.9, since this value is ...
... with integrating spheres. This method allows one to determine the absorption and the reduced scattering coefficients of a turbid media from the measured values of the total transmittance and the diffuse reflectance. In these calculations the anisotropy factor can be fixed as 0.9, since this value is ...
Chemistry SOL Review Test
... 16) Describe Thomson’s atom model. Electron are like raisins surrounded by a soup of positive charge to balance the electrons' negative charges (Plum Pudding Model). 17) What experiment did Thomson do? The cathode rays tube 18) What was his model called? Plum Pudding Model 19) Describe Rutherford’s ...
... 16) Describe Thomson’s atom model. Electron are like raisins surrounded by a soup of positive charge to balance the electrons' negative charges (Plum Pudding Model). 17) What experiment did Thomson do? The cathode rays tube 18) What was his model called? Plum Pudding Model 19) Describe Rutherford’s ...
Subwavelength transportation of light with atomic resonances
... waveguide crossings [18], high Q factor resonators [19], and ultrabroadband multimode interference (MMI) couplers [20] are demonstrated in subwavelength structures. In parallel, nanoscale waveguides that confine, guide, and manipulate the electromagnetic energy on subwavelength scales have been acti ...
... waveguide crossings [18], high Q factor resonators [19], and ultrabroadband multimode interference (MMI) couplers [20] are demonstrated in subwavelength structures. In parallel, nanoscale waveguides that confine, guide, and manipulate the electromagnetic energy on subwavelength scales have been acti ...
Building the sense of math in physics activities
... the object and v is its velocity through the fluid. (This is actually correct up to a dimensionless factor. For this problem take Re to be the ratio of these two forces.) B.1 Write an equation for the Reynolds number for this example, simplifying the equation as much as possible (e.g., cancelling fa ...
... the object and v is its velocity through the fluid. (This is actually correct up to a dimensionless factor. For this problem take Re to be the ratio of these two forces.) B.1 Write an equation for the Reynolds number for this example, simplifying the equation as much as possible (e.g., cancelling fa ...
Lab 1
... •Describe some physical properties of the elements you observe. •Categorize an element as a metal , nonmetal or metalloid from its physical properties. •Given the complete symbol of an atom, determine its mass number, atomic number, and the number of protons, neutrons, and electrons. ...
... •Describe some physical properties of the elements you observe. •Categorize an element as a metal , nonmetal or metalloid from its physical properties. •Given the complete symbol of an atom, determine its mass number, atomic number, and the number of protons, neutrons, and electrons. ...
avogadro exam 2001 - University of Waterloo
... The results of Student A are more accurate but less precise. ...
... The results of Student A are more accurate but less precise. ...
Thermodynamics - WordPress.com
... During phase changes the temp remains constant (even though heat is being added or removed from the system) so no change in the average kinetic energy (Ek) of the molecules occurs. According to the chemical bonding theory energy is required to overcome forces or bonds that hold particles together, s ...
... During phase changes the temp remains constant (even though heat is being added or removed from the system) so no change in the average kinetic energy (Ek) of the molecules occurs. According to the chemical bonding theory energy is required to overcome forces or bonds that hold particles together, s ...
Atomic matter of nonzero-momentum Bose-Einstein condensation and orbital current order
... Confining bosonic atoms in an optical lattice can bring out different and new physics beyond the standard BoseEinstein condensation 共BEC兲 observed in a single trap 关1,2兴. The superfluid–Mott-insulator experiment on an optical lattice 关3兴, based on an early theoretical idea 关4,5兴, demonstrated one su ...
... Confining bosonic atoms in an optical lattice can bring out different and new physics beyond the standard BoseEinstein condensation 共BEC兲 observed in a single trap 关1,2兴. The superfluid–Mott-insulator experiment on an optical lattice 关3兴, based on an early theoretical idea 关4,5兴, demonstrated one su ...
Phys. Rev. Lett. 104, 043002 (2010)
... to the regime of multiple Rydberg atoms, such excitation crystals provide a suitable initial state to realize quantum random walks [17]. We show that the crystalline correlations can be mapped onto a propagating light beam and be detected via photon correlation measurements as a pulse train of singl ...
... to the regime of multiple Rydberg atoms, such excitation crystals provide a suitable initial state to realize quantum random walks [17]. We show that the crystalline correlations can be mapped onto a propagating light beam and be detected via photon correlation measurements as a pulse train of singl ...
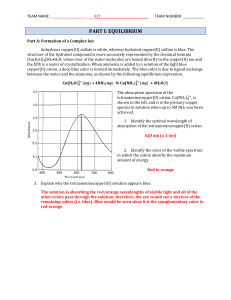
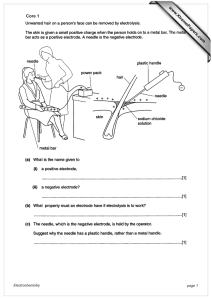
Core 1 www.XtremePapers.com Electrochemistry page 1
... copper formed or iron dissolves zinc not displaced or iron does not react with zinc ions ...
... copper formed or iron dissolves zinc not displaced or iron does not react with zinc ions ...
Development of the Modern Atomic Theory
... • In order to determine how many neutrons are in the nucleus of an atom simply subtract: ...
... • In order to determine how many neutrons are in the nucleus of an atom simply subtract: ...
1. some basic concepts of chemistry
... ratio, i.e. 16:32 or 1: 2. 4. Gay Lussac’s Law of Gaseous Volumes: This law was proposed by Gay Lussac. It states that when gases combine to form gaseous products, their volumes are in simple whole number ratio at constant temperature and pressure. Illustration: H2 combines with O2 to form water vap ...
... ratio, i.e. 16:32 or 1: 2. 4. Gay Lussac’s Law of Gaseous Volumes: This law was proposed by Gay Lussac. It states that when gases combine to form gaseous products, their volumes are in simple whole number ratio at constant temperature and pressure. Illustration: H2 combines with O2 to form water vap ...
File
... present ionizes into one H+ ion and one Cl− ion. The concentration of OH− can then be determined from [H+] and Kw . Step 2: Solve. [H+] = 2.0 × 10−3 M Kw = [H+][OH-] = 1.0 × 10−14 [OH-] = Kw/[H+] = 1.0 x 10-14 / 2.0 x 10-3 = 5.0 x 10-12 M Step 3: Think about your result. [H+] is much higher than [O ...
... present ionizes into one H+ ion and one Cl− ion. The concentration of OH− can then be determined from [H+] and Kw . Step 2: Solve. [H+] = 2.0 × 10−3 M Kw = [H+][OH-] = 1.0 × 10−14 [OH-] = Kw/[H+] = 1.0 x 10-14 / 2.0 x 10-3 = 5.0 x 10-12 M Step 3: Think about your result. [H+] is much higher than [O ...
Chem 173: Final Exam Review Short Answer and Problems 1
... 1.40 mol of yttrium metal and 1.40 mol of molecular oxygen are allowed to react according to the following equation: 4 Y (s) + 3 O2 (g) Ÿ 2 Y2O3 (s) In this reaction the limiting reactant is _______________ . If 0.475 mol of Y2O3 is actually recovered in an experiment, the percent yield is _________ ...
... 1.40 mol of yttrium metal and 1.40 mol of molecular oxygen are allowed to react according to the following equation: 4 Y (s) + 3 O2 (g) Ÿ 2 Y2O3 (s) In this reaction the limiting reactant is _______________ . If 0.475 mol of Y2O3 is actually recovered in an experiment, the percent yield is _________ ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.