Year 11 C2 Mock Exam Revision Questions
... sentences below which are about this compound. Phosphorus trifluoride is made up of phosphorus and fluorine ................................ These are joined together by sharing pairs of ............................................... to form ...
... sentences below which are about this compound. Phosphorus trifluoride is made up of phosphorus and fluorine ................................ These are joined together by sharing pairs of ............................................... to form ...
Selective photodissociation of tailored molecular - Beilstein
... shown in Figure 5 (red line). Even in the absence of any UV light, one can see fragments which we attribute to the IR laser desorption (black line in Figure 5). The strongest beam depletion was observed when the 266 nm laser pulse arrived about 600 µs before the VUV ionizing laser pulse, i.e., short ...
... shown in Figure 5 (red line). Even in the absence of any UV light, one can see fragments which we attribute to the IR laser desorption (black line in Figure 5). The strongest beam depletion was observed when the 266 nm laser pulse arrived about 600 µs before the VUV ionizing laser pulse, i.e., short ...
Rejection of two-photon fluorescence background in
... where ~ρ and z are coordinates relative to the laser beam focus (i.e. the surface of the sample is located at a negative z). This is the surviving portion of the laser intensity that has not incured scattering on its Rpassage to depth z. The lateral beam profile is written as PSF(~ρ , z), which we n ...
... where ~ρ and z are coordinates relative to the laser beam focus (i.e. the surface of the sample is located at a negative z). This is the surviving portion of the laser intensity that has not incured scattering on its Rpassage to depth z. The lateral beam profile is written as PSF(~ρ , z), which we n ...
Chemistry1100 Practice Exam 4 Choose the best answer for
... Suppose that 2.500 g of each reactant is added together. Calculate the number of grams of the KNO3 that will be produced. Then identify the Limiting Reagent and the Excess Reagent. 0.0151 mol KNO3 from Pb(NO3)3 so the lead nitrate is the limiting reagent 0.0446 mol KNO3 from KOH so the KOH is the ex ...
... Suppose that 2.500 g of each reactant is added together. Calculate the number of grams of the KNO3 that will be produced. Then identify the Limiting Reagent and the Excess Reagent. 0.0151 mol KNO3 from Pb(NO3)3 so the lead nitrate is the limiting reagent 0.0446 mol KNO3 from KOH so the KOH is the ex ...
Encircled energy factor in impulse response functions of
... that the intensity distribution in the focal region will be symmetrical about the focal plane. But subsequent studies show that the principal maximum of the diffraction pattern may not be at the geometrical focuses and moves towards the aperture depending upon the Fresnel number (N) of the system. T ...
... that the intensity distribution in the focal region will be symmetrical about the focal plane. But subsequent studies show that the principal maximum of the diffraction pattern may not be at the geometrical focuses and moves towards the aperture depending upon the Fresnel number (N) of the system. T ...
Elements, Compounds, and Mixtures
... The Atom An atom consists of a Nucleus ( protons and neutrons) electrons in space around the nucleus. ...
... The Atom An atom consists of a Nucleus ( protons and neutrons) electrons in space around the nucleus. ...
Monodisperse FePt Nanoparticles and Ferromagnetic FePt
... the HRSEM images of both surface (Fig. 1C) and cross section (not shown in figure) of the assembly show that the particles are well separated with no agglomeration occurring. Interparticle spacings, however, are reduced from ⬃4 to ⬃2 nm, as indicated by TEM, HRSEM, and small angle x-ray scattering e ...
... the HRSEM images of both surface (Fig. 1C) and cross section (not shown in figure) of the assembly show that the particles are well separated with no agglomeration occurring. Interparticle spacings, however, are reduced from ⬃4 to ⬃2 nm, as indicated by TEM, HRSEM, and small angle x-ray scattering e ...
Why do molecules form the way they do?
... - The potential thermodynamic energy of a reacting system - The potential energy stored (as heat) in chemical bonds - Exothermic reactions have negative DHrxn values -Typically (but not always) spontaneous reactions have negative values of DHrxn (the heat term is added to the products side) - We exp ...
... - The potential thermodynamic energy of a reacting system - The potential energy stored (as heat) in chemical bonds - Exothermic reactions have negative DHrxn values -Typically (but not always) spontaneous reactions have negative values of DHrxn (the heat term is added to the products side) - We exp ...
ChemQuiz_QntativeChem
... Separate samples of two gases, each containing a pure substance, are found to have the same density under the same conditions of temperature and pressure. Which statement about these two samples must be correct? ...
... Separate samples of two gases, each containing a pure substance, are found to have the same density under the same conditions of temperature and pressure. Which statement about these two samples must be correct? ...
Ca(ii), Cd(ii), Cu(ii) and Pb(ii)
... University of Lodz, Tamka 12, 91-403 Łódź, Poland. E-mail: [email protected] ...
... University of Lodz, Tamka 12, 91-403 Łódź, Poland. E-mail: [email protected] ...
Congratulations! You have signed up for AP Chemistry for this year
... 400 B.C.—Greeks—proposed all matter was make up of 4 “elements” : fire, earth, water and air Democritus—first to use the term atomos to describe the ultimate, smallest particles of matter Next 2,000 years—alchemy—a pseudoscience where people thought they could turn metals into gold. Some good chemis ...
... 400 B.C.—Greeks—proposed all matter was make up of 4 “elements” : fire, earth, water and air Democritus—first to use the term atomos to describe the ultimate, smallest particles of matter Next 2,000 years—alchemy—a pseudoscience where people thought they could turn metals into gold. Some good chemis ...
CHAPTER 8: CHEMICAL COMPOSITION
... then the (weighted average) atomic mass of carbon is calculated as follows: (12.000000 amu)(0.9893) + (13.003354 amu)(0.0107) = 12.01 amu So how many carbon atoms are present in 12.01 grams of carbon? This number was determined experimentally to be 6.022×1023. – It was named Avogadro’s number, to ho ...
... then the (weighted average) atomic mass of carbon is calculated as follows: (12.000000 amu)(0.9893) + (13.003354 amu)(0.0107) = 12.01 amu So how many carbon atoms are present in 12.01 grams of carbon? This number was determined experimentally to be 6.022×1023. – It was named Avogadro’s number, to ho ...
Ultra cold atoms and Bose-Einstein condensation for quantum
... photon scattering, which limits the final velocity to a few times the elementary step in velocity diffusion, the recoil velocity vrec , see Eq. (2). The recoil temperature is usually much less than the Doppler temperature, for example 2.4 µK for sodium and 200 nK for caesium. 3.3 Sub-recoil cooling To ...
... photon scattering, which limits the final velocity to a few times the elementary step in velocity diffusion, the recoil velocity vrec , see Eq. (2). The recoil temperature is usually much less than the Doppler temperature, for example 2.4 µK for sodium and 200 nK for caesium. 3.3 Sub-recoil cooling To ...
Solution
... A gas first expands isothermally against a vacuum (process I) and then is compressed isothermally and reversibly to its original volume and temperature (process II). Determine the value (0, < 0, > 0) for each of the quantities below for process I, process II, and the overall cyclic process (I+II), a ...
... A gas first expands isothermally against a vacuum (process I) and then is compressed isothermally and reversibly to its original volume and temperature (process II). Determine the value (0, < 0, > 0) for each of the quantities below for process I, process II, and the overall cyclic process (I+II), a ...
Final Review 2006
... ____ 30. Which observation does NOT indicate that a chemical reaction has occurred? a. formation of a precipitate c. evolution of heat and light b. production of a gas d. change in total mass of substances ____ 31. A solid produced by a chemical reaction in solution that separates from the solution ...
... ____ 30. Which observation does NOT indicate that a chemical reaction has occurred? a. formation of a precipitate c. evolution of heat and light b. production of a gas d. change in total mass of substances ____ 31. A solid produced by a chemical reaction in solution that separates from the solution ...
... to coalesce, as in Figure 5a below. To coalesce into one filament, the two initial filaments must each have a power less than about 1.35*Pcr and be separated by less than a critical distance defined by 10 times the filament waist size [3]. If the power contained in each filament is more than this, t ...
Chem 310 Lectures by: Dr. Muhammad D. Bala Office: Block H, 3
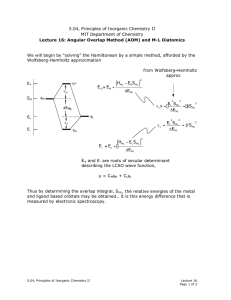
... “Any non-linear molecular system in a degenerate electronic state will be unstable and will undergo distortion to form a system of lower symmetry and lower energy thereby removing the degeneracy” In simple terms it means that no nonlinear molecule can be stable in a degenerate electronic state. The ...
... “Any non-linear molecular system in a degenerate electronic state will be unstable and will undergo distortion to form a system of lower symmetry and lower energy thereby removing the degeneracy” In simple terms it means that no nonlinear molecule can be stable in a degenerate electronic state. The ...
Producing Squeezed Input States for an Atomic Clock Using an Optical Cavity.
... space leads to a loss of squeezing performance that is independent of atom number [12]. Thus it does not modify the fundamental scaling of the achievable signal-to-noise ratio with atom number. In contrast, Raman scattering which flips the clock spin generically reduces the achievable spin squeezing ...
... space leads to a loss of squeezing performance that is independent of atom number [12]. Thus it does not modify the fundamental scaling of the achievable signal-to-noise ratio with atom number. In contrast, Raman scattering which flips the clock spin generically reduces the achievable spin squeezing ...
BSPH 111 - Refresher Chemistry
... An atom consists of a nucleus of protons and neutrons, surrounded by electrons. Each of the elements in the periodic table is classified according to its atomic number, which is the number of protons in that element's nucleus. Protons have a charge of +1, electrons have a charge of -1, and neutrons ...
... An atom consists of a nucleus of protons and neutrons, surrounded by electrons. Each of the elements in the periodic table is classified according to its atomic number, which is the number of protons in that element's nucleus. Protons have a charge of +1, electrons have a charge of -1, and neutrons ...
WEEK
... Radioactive decay, fission, and fusion are nuclear reactions which involve changes in the nucleus of an atom releasing larger amounts of energy per mass than in chemical reactions. ...
... Radioactive decay, fission, and fusion are nuclear reactions which involve changes in the nucleus of an atom releasing larger amounts of energy per mass than in chemical reactions. ...
Chapter 3 - Stoichiometry
... number of particles. Since atoms and molecules are very small, counting them would be very difficult. But we have to invent a unit such that this standard molecular amount is a specific number of particles which can allow us to predict its mass and/or volume. The standard molecular amount is called ...
... number of particles. Since atoms and molecules are very small, counting them would be very difficult. But we have to invent a unit such that this standard molecular amount is a specific number of particles which can allow us to predict its mass and/or volume. The standard molecular amount is called ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.