Java Based Distributed Learning Platform
... oxidation-reduction cycles (ORC) procedure [18,19] is a better way to produce SERS-active metal substrates because a controllable and reproduced surface roughness can be easily generated [20,21]. In this work, we use an electrochemical ORC roughening procedure ...
... oxidation-reduction cycles (ORC) procedure [18,19] is a better way to produce SERS-active metal substrates because a controllable and reproduced surface roughness can be easily generated [20,21]. In this work, we use an electrochemical ORC roughening procedure ...
L. T. Zhuravlev, The surface chemistry of amorphous silica
... A review article is presented of the research results obtained by the author on the properties of amorphous silica surface. It has been shown that in any description of the surface silica the hydroxylation of the surface is of critical importance. An analysis was made of the processes of dehydration ...
... A review article is presented of the research results obtained by the author on the properties of amorphous silica surface. It has been shown that in any description of the surface silica the hydroxylation of the surface is of critical importance. An analysis was made of the processes of dehydration ...
Prospects of Emerging Engineered oxide nanomaterials and their
... certain chemical or physical processes or both which could be used in numerous thrust areas. An entirely new concept of unique features in terms of sensing, optical-, electro-, photo-, and magnetic-properties and their applications including thermal stability and chemical resistance of MON depends o ...
... certain chemical or physical processes or both which could be used in numerous thrust areas. An entirely new concept of unique features in terms of sensing, optical-, electro-, photo-, and magnetic-properties and their applications including thermal stability and chemical resistance of MON depends o ...
Electronic transport at semiconductor
... three-dimensional (3D) crystals (consider insulating diamond and conducting graphite, both of which are consisted of carbon atoms). It is also true of the 2D periodic structures at crystal surfaces. What would Brattain and Bardeen have discovered with their two-point probe, had they had access to th ...
... three-dimensional (3D) crystals (consider insulating diamond and conducting graphite, both of which are consisted of carbon atoms). It is also true of the 2D periodic structures at crystal surfaces. What would Brattain and Bardeen have discovered with their two-point probe, had they had access to th ...
Mechanisms and energetics of surface reactions at the copper
... Even if the initial oxygen coverage would be very low (as can be achieved experimentally by mechanical polishing and chemical reduction (Clendening and Campbell 1989)), the oxide layer could grow also in anoxic water due to the cleavage of water molecules. By cleaving the water molecules in S1, ini ...
... Even if the initial oxygen coverage would be very low (as can be achieved experimentally by mechanical polishing and chemical reduction (Clendening and Campbell 1989)), the oxide layer could grow also in anoxic water due to the cleavage of water molecules. By cleaving the water molecules in S1, ini ...
Manual Physical Chemistry III
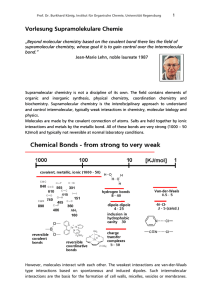
... The molecules of liquids attract each other by cohesive forces resulting into small distances between the molecules (on the order of 0.1 nm). Thus the compressibility of liquids is lower than that of gas, while the density is much higher. On the other hand, these cohesive forces are not strong enoug ...
... The molecules of liquids attract each other by cohesive forces resulting into small distances between the molecules (on the order of 0.1 nm). Thus the compressibility of liquids is lower than that of gas, while the density is much higher. On the other hand, these cohesive forces are not strong enoug ...
Ultrasonic spray deposition for production of organic solar cells
... improvements due to increased wetting of a single deposited layer from p-xylene while Fig. 2b shows the dominant coffee-stained texture [19] of the chlorobenzene-deposited layer. The lower surface tension of the p-xylene solution enhances wetting and, hence, uniting of drops on the substrate. This a ...
... improvements due to increased wetting of a single deposited layer from p-xylene while Fig. 2b shows the dominant coffee-stained texture [19] of the chlorobenzene-deposited layer. The lower surface tension of the p-xylene solution enhances wetting and, hence, uniting of drops on the substrate. This a ...
Self-assembled monolayer

Self-assembled monolayers (SAM) of organic molecules are molecular assemblies formed spontaneously on surfaces by adsorption and are organized into more or less large ordered domains. In some cases molecules that form the monolayer do not interact strongly with the substrate. This is the case for instance of the two-dimensional supramolecular networks of e.g. Perylene-tetracarboxylicacid-dianhydride (PTCDA) on gold or of e.g. porphyrins on highly oriented pyrolitic graphite (HOPG). In other cases the molecules possess a head group that has a strong affinity to the substrate and anchors the molecule to it. Such a SAM consisting of a head group, tail and functional end group is depicted in Figure 1. Common head groups include thiols, silanes, phosphonates, etc.SAMs are created by the chemisorption of ""head groups"" onto a substrate from either the vapor or liquid phase followed by a slow organization of ""tail groups"". Initially, at small molecular density on the surface, adsorbate molecules form either a disordered mass of molecules or form an ordered two-dimensional ""lying down phase"", and at higher molecular coverage, over a period of minutes to hours, begin to form three-dimensional crystalline or semicrystalline structures on the substrate surface. The ""head groups"" assemble together on the substrate, while the tail groups assemble far from the substrate. Areas of close-packed molecules nucleate and grow until the surface of the substrate is covered in a single monolayer.Adsorbate molecules adsorb readily because they lower the surface free-energy of the substrate and are stable due to the strong chemisorption of the ""head groups."" These bonds create monolayers that are more stable than the physisorbed bonds of Langmuir–Blodgett films. A Trichlorosilane based ""head group"", for example in a FDTS molecule reacts with an hydroxyl group on a substrate, and forms very stable, covalent bond [R-Si-O-substrate] with an energy of 452 kJ/mol. Thiol-metal bonds, that are on the order of 100 kJ/mol, making the bond a fairly stable in a variety of temperature, solvents, and potentials. The monolayer packs tightly due to van der Waals interactions, thereby reducing its own free energy. The adsorption can be described by the Langmuir adsorption isotherm if lateral interactions are neglected. If they cannot be neglected, the adsorption is better described by the Frumkin isotherm.