doc_296
... A nanocapsule is any nanoparticle that consists of a shell and a space, in which desired substances may be placed. Technologies for microencapsulating materials have been around for several years, primarily for applications involving minimisation of hygroscopy and chemical interactions, elimination ...
... A nanocapsule is any nanoparticle that consists of a shell and a space, in which desired substances may be placed. Technologies for microencapsulating materials have been around for several years, primarily for applications involving minimisation of hygroscopy and chemical interactions, elimination ...
File
... If you know how many moles of a substance you have, you can find its total mass using the molar mass of that substance. Ex: What is the mass of 2.42 mol of H2O? ...
... If you know how many moles of a substance you have, you can find its total mass using the molar mass of that substance. Ex: What is the mass of 2.42 mol of H2O? ...
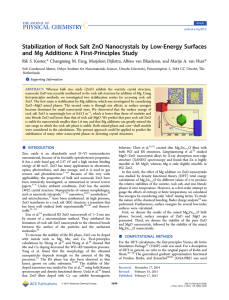
Stabilization of Rock Salt ZnO Nanocrystals by Low
... The average charges on the Mg, Zn, and O atoms vary more or less linearly with x. This change is very small for the cations (∼0.02 e), and none of the cations within a cell has a charge that deviates much from the average. The average charge on the oxygen atoms, on the other hand, varies from −1.2 e ...
... The average charges on the Mg, Zn, and O atoms vary more or less linearly with x. This change is very small for the cations (∼0.02 e), and none of the cations within a cell has a charge that deviates much from the average. The average charge on the oxygen atoms, on the other hand, varies from −1.2 e ...
Optical modeling of finite element surface
... Surface interferogram files are CODEV native and are two-dimensional data sets that are assigned as deviations to an optical surface. The data is assumed normal to the optical surface requiring finite element displacements to be converted into surface normal displacements. Interferogram file data ma ...
... Surface interferogram files are CODEV native and are two-dimensional data sets that are assigned as deviations to an optical surface. The data is assumed normal to the optical surface requiring finite element displacements to be converted into surface normal displacements. Interferogram file data ma ...
Method of making an epitaxial gallium arsenide semiconductor
... monocrystalline silicon substrate having a major surface technologies for producing and processing its ingot are most advanced in the ?eld of semiconductor materials. 45 inclined at an off angle between 05° and 5° with respect to (100), a step of forming at least one intermediate layer In this case, ...
... monocrystalline silicon substrate having a major surface technologies for producing and processing its ingot are most advanced in the ?eld of semiconductor materials. 45 inclined at an off angle between 05° and 5° with respect to (100), a step of forming at least one intermediate layer In this case, ...
Chemical Dynamics at Surfaces
... – as well as the modern experimental methods used to probe the limits of this understanding. ...
... – as well as the modern experimental methods used to probe the limits of this understanding. ...
Heterogeneous Nanonucleants - Manuscript - FINAL
... Surfaces exhibiting topological features have also been applied to controlling crystallisation of organic electronics. Bao’s group reported the use of rough patches of octadecyltriethoxysilane (OTS) functionalized dielectric surfaces (average roughness 10-15nm) for templating single-crystal growth o ...
... Surfaces exhibiting topological features have also been applied to controlling crystallisation of organic electronics. Bao’s group reported the use of rough patches of octadecyltriethoxysilane (OTS) functionalized dielectric surfaces (average roughness 10-15nm) for templating single-crystal growth o ...
Self-assembled monolayer

Self-assembled monolayers (SAM) of organic molecules are molecular assemblies formed spontaneously on surfaces by adsorption and are organized into more or less large ordered domains. In some cases molecules that form the monolayer do not interact strongly with the substrate. This is the case for instance of the two-dimensional supramolecular networks of e.g. Perylene-tetracarboxylicacid-dianhydride (PTCDA) on gold or of e.g. porphyrins on highly oriented pyrolitic graphite (HOPG). In other cases the molecules possess a head group that has a strong affinity to the substrate and anchors the molecule to it. Such a SAM consisting of a head group, tail and functional end group is depicted in Figure 1. Common head groups include thiols, silanes, phosphonates, etc.SAMs are created by the chemisorption of ""head groups"" onto a substrate from either the vapor or liquid phase followed by a slow organization of ""tail groups"". Initially, at small molecular density on the surface, adsorbate molecules form either a disordered mass of molecules or form an ordered two-dimensional ""lying down phase"", and at higher molecular coverage, over a period of minutes to hours, begin to form three-dimensional crystalline or semicrystalline structures on the substrate surface. The ""head groups"" assemble together on the substrate, while the tail groups assemble far from the substrate. Areas of close-packed molecules nucleate and grow until the surface of the substrate is covered in a single monolayer.Adsorbate molecules adsorb readily because they lower the surface free-energy of the substrate and are stable due to the strong chemisorption of the ""head groups."" These bonds create monolayers that are more stable than the physisorbed bonds of Langmuir–Blodgett films. A Trichlorosilane based ""head group"", for example in a FDTS molecule reacts with an hydroxyl group on a substrate, and forms very stable, covalent bond [R-Si-O-substrate] with an energy of 452 kJ/mol. Thiol-metal bonds, that are on the order of 100 kJ/mol, making the bond a fairly stable in a variety of temperature, solvents, and potentials. The monolayer packs tightly due to van der Waals interactions, thereby reducing its own free energy. The adsorption can be described by the Langmuir adsorption isotherm if lateral interactions are neglected. If they cannot be neglected, the adsorption is better described by the Frumkin isotherm.