Atoms, Molecules and Optical Physics 1 and 2
... active and highly productive research in physics. And in spite of, or perhaps even because of its remarkable history the field continues to constitute an indispensable basis for any more profound understanding of nearly all branches of modern physics, physical chemistry and partially even biological ...
... active and highly productive research in physics. And in spite of, or perhaps even because of its remarkable history the field continues to constitute an indispensable basis for any more profound understanding of nearly all branches of modern physics, physical chemistry and partially even biological ...
The nature of light - FIU Faculty Websites
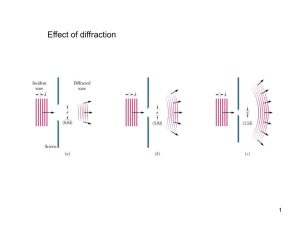
... that light was made up of waves vibrating up and down perpendicular to the direction of the light travels. This became known as 'Huygens' Principle'. Huygen, suggested that light wave peaks form surfaces like the layers of an onion. In a vacuum, or other uniform mediums, the light waves are spherica ...
... that light was made up of waves vibrating up and down perpendicular to the direction of the light travels. This became known as 'Huygens' Principle'. Huygen, suggested that light wave peaks form surfaces like the layers of an onion. In a vacuum, or other uniform mediums, the light waves are spherica ...
Optical absorption cross section of quantum dots
... section determines the maximum optical gain, which can be obtained from a system of dots if the occupation of the states participating in the transition are fully inverted (i.e. all the upper states are filled, all the lower states are empty). The size, shape and composition of the dot and its surro ...
... section determines the maximum optical gain, which can be obtained from a system of dots if the occupation of the states participating in the transition are fully inverted (i.e. all the upper states are filled, all the lower states are empty). The size, shape and composition of the dot and its surro ...
RAY OPTICS notes
... The distances measured in the same direction as the incident light are taken as positive and those measured in the direction opposite to the direction of incident light are taken as negative . ...
... The distances measured in the same direction as the incident light are taken as positive and those measured in the direction opposite to the direction of incident light are taken as negative . ...
Learning Outcomes
... If light of one colour is absorbed, then the complementary colour will be observed. UV and visible absorption of transition metal complexes The effects of d-d transitions can be studied using ultra-violet and visible absorption spectroscopy. Ultra-violet and visible absorption spectroscopy involves ...
... If light of one colour is absorbed, then the complementary colour will be observed. UV and visible absorption of transition metal complexes The effects of d-d transitions can be studied using ultra-violet and visible absorption spectroscopy. Ultra-violet and visible absorption spectroscopy involves ...
Lecture 11
... M matricies can be used to propagate either left to right or right to left (input to output or output to input). The output to input formulation is both a bit more numerically friendly, and much more useful if you want to explore the field distribution inside the dielectric stack. High reflectivity ...
... M matricies can be used to propagate either left to right or right to left (input to output or output to input). The output to input formulation is both a bit more numerically friendly, and much more useful if you want to explore the field distribution inside the dielectric stack. High reflectivity ...
21. Specific rotation of sugar solution
... the direction axis. Cross-wave polarization in general, stands for disorder of axis-symmetry according to the spreading direction. Polarized light is divided into categories by the projection of the E-vector endpoint trace on the plane, which is perpendicular to the direction of light propagation. ...
... the direction axis. Cross-wave polarization in general, stands for disorder of axis-symmetry according to the spreading direction. Polarized light is divided into categories by the projection of the E-vector endpoint trace on the plane, which is perpendicular to the direction of light propagation. ...
The Energy and Geometrical Structure of Molecules
... mode), where its skeletal structure bends back and forth. This means that, by absorbing this infrared light, CO2 is excited to its vibrationally excited state, as schematically shown in Fig. 1.6. The energy gained by the infrared photoabsorption may be lost from the vibrationally exited molecule thr ...
... mode), where its skeletal structure bends back and forth. This means that, by absorbing this infrared light, CO2 is excited to its vibrationally excited state, as schematically shown in Fig. 1.6. The energy gained by the infrared photoabsorption may be lost from the vibrationally exited molecule thr ...
1999 Advanced Placement Chemistry Exam Section I: Multiple
... (C) all points on the curve between Q and S (A) The pressure on the walls of the balloon in(D) all points on the curve between R and T creases with increasing temperature. (E) no point on the curve (B) The difference in temperature between the air inside and outside the balloon produces ... C10H12O4 ...
... (C) all points on the curve between Q and S (A) The pressure on the walls of the balloon in(D) all points on the curve between R and T creases with increasing temperature. (E) no point on the curve (B) The difference in temperature between the air inside and outside the balloon produces ... C10H12O4 ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.