chemical reaction
... • a substance that _________ the reaction rate ________ being used up in the reaction. • A substance that ______ up a reaction without being permanently changed. NOT A ___________!! • Ex: __________ speed up reactions in your body. ...
... • a substance that _________ the reaction rate ________ being used up in the reaction. • A substance that ______ up a reaction without being permanently changed. NOT A ___________!! • Ex: __________ speed up reactions in your body. ...
Unit 6 Naming Binary Compounds
... in multiple compounds until the very end; they might take care of themselves. ...
... in multiple compounds until the very end; they might take care of themselves. ...
Physical and Chemical Changes
... There are TWO types of physical changes. • Reversible Change: A change that can be changed back to its original form. ...
... There are TWO types of physical changes. • Reversible Change: A change that can be changed back to its original form. ...
2 (aq)
... Designates a reactant or product in the liquid state: placed after the formula Designates a reactant or product in the gaseous state; placed after the formula Designates an aqueous solution; the substance is dissolved in water; placed after the formula Indicates that heat is supplied to the reaction ...
... Designates a reactant or product in the liquid state: placed after the formula Designates a reactant or product in the gaseous state; placed after the formula Designates an aqueous solution; the substance is dissolved in water; placed after the formula Indicates that heat is supplied to the reaction ...
VCAA Study Design - Chemistry Education Association
... • lack of awareness of the differences between discharging and recharging in terms of the direction of electron flow, and that electrons always move from the site of oxidation (anode) to the site of reduction (cathode) • inability to correctly explain the changes in the rates of the forward and reve ...
... • lack of awareness of the differences between discharging and recharging in terms of the direction of electron flow, and that electrons always move from the site of oxidation (anode) to the site of reduction (cathode) • inability to correctly explain the changes in the rates of the forward and reve ...
Chemistry Essentials Unit 2
... Two or more substances that occupy the same container without interacting with each other No chemical bonds between components of the mixture Variable composition from sample to sample or within a sample Properties are usually a blend of the properties of the components in the mixture No definite fo ...
... Two or more substances that occupy the same container without interacting with each other No chemical bonds between components of the mixture Variable composition from sample to sample or within a sample Properties are usually a blend of the properties of the components in the mixture No definite fo ...
FoundationsofChemistryppt
... 1. The atoms in all objects are the same. 2. You cannot always tell by an object’s appearance whether it is made of more than one type of atom. 3. The weight of a material never changes, regardless of where it is. 4. Boiling is one method used to separate parts of a mixture. ...
... 1. The atoms in all objects are the same. 2. You cannot always tell by an object’s appearance whether it is made of more than one type of atom. 3. The weight of a material never changes, regardless of where it is. 4. Boiling is one method used to separate parts of a mixture. ...
Section 4.6: Double Displacement Reactions
... result is a new compound and a new element. In a double displacement reaction, two elements trade places to form two new compounds. The result is that the two compounds react to form two new compounds. 2. (a) The reaction is a double displacement reaction. (b) The reaction is a single displacement r ...
... result is a new compound and a new element. In a double displacement reaction, two elements trade places to form two new compounds. The result is that the two compounds react to form two new compounds. 2. (a) The reaction is a double displacement reaction. (b) The reaction is a single displacement r ...
PHYSICAL PROPERTIES - can observe w/o changing the
... Examples: burning, dissolving something in an acid, letting iron rust, letting silver tarnish, mixing vinegar and baking soda, cooking an egg Also called a CHEMICAL REACTION (5 signs to watch for) formation of an odor, change in temp, formation of a precipitate, change in color, formation of gas ...
... Examples: burning, dissolving something in an acid, letting iron rust, letting silver tarnish, mixing vinegar and baking soda, cooking an egg Also called a CHEMICAL REACTION (5 signs to watch for) formation of an odor, change in temp, formation of a precipitate, change in color, formation of gas ...
Chapter-1-Intro - Mister Chemistry Welcomes You!
... a concise verbal or mathematical statement of a relationship between phenomena that is always the same under the same conditions ...
... a concise verbal or mathematical statement of a relationship between phenomena that is always the same under the same conditions ...
H 2
... contain Dioxide encounter iniscarbon. your burning. (CO daily. car, 2) the The chemical equation is gas balanced natural The This reaction reaction In gas this other that of case, allows organic heats product it is us your methane compounds toisrelease home, Water coal, energy (H (CH with oil ) from ...
... contain Dioxide encounter iniscarbon. your burning. (CO daily. car, 2) the The chemical equation is gas balanced natural The This reaction reaction In gas this other that of case, allows organic heats product it is us your methane compounds toisrelease home, Water coal, energy (H (CH with oil ) from ...
chem equation Pkt Student2
... e) remember the rules for writing formulas for molecular compounds (______________) • Only NONMETALS! f) remember the formula for water, ________ • HOH = hydrogen hydroxide ...
... e) remember the rules for writing formulas for molecular compounds (______________) • Only NONMETALS! f) remember the formula for water, ________ • HOH = hydrogen hydroxide ...
Chapter 8 Chemical Equations and Reactions
... iv) List four kinds of single-displacement reactions and three kinds of double-displacement reactions. v) Predict the products of simple reactions given the reactants. ...
... iv) List four kinds of single-displacement reactions and three kinds of double-displacement reactions. v) Predict the products of simple reactions given the reactants. ...
Reactions of Metals and Their Compounds
... Chemical and Physical Changes What is the important difference between and CHEMICAL and PHYSICAL change? PHYSICAL = the substance does not become a ...
... Chemical and Physical Changes What is the important difference between and CHEMICAL and PHYSICAL change? PHYSICAL = the substance does not become a ...
Mole Ratio and Mass IP
... Use the chemical equation to write a mole:mole ratio that allows you to convert from one chemical to another. This is a Magic Moment: it is the ONLY time you can go from one chemical to another! I use a DIAMOND to mark this kind of factor. ...
... Use the chemical equation to write a mole:mole ratio that allows you to convert from one chemical to another. This is a Magic Moment: it is the ONLY time you can go from one chemical to another! I use a DIAMOND to mark this kind of factor. ...
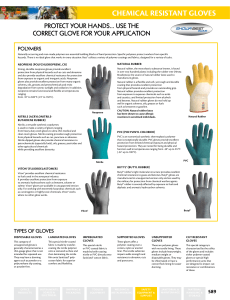
chemical reSiStant GloveS
... protection from physical hazards such as cuts and abrasions and also provide excellent chemical resistance for protection from exposure to organic and inorganic acids. Neoprene gloves also provide excellent protection from many organic solvents, oils, greases and petrochemicals and resist degradatio ...
... protection from physical hazards such as cuts and abrasions and also provide excellent chemical resistance for protection from exposure to organic and inorganic acids. Neoprene gloves also provide excellent protection from many organic solvents, oils, greases and petrochemicals and resist degradatio ...
Chemical Equations
... f) remember the formula for water, ________ • HOH = hydrogen hydroxide 3. Write a balanced chemical equation by adding_____________________, NOT subscripts (this will require trial and error, the following guidelines may be helpful) a) balance the different types of atoms ________________ b) first, ...
... f) remember the formula for water, ________ • HOH = hydrogen hydroxide 3. Write a balanced chemical equation by adding_____________________, NOT subscripts (this will require trial and error, the following guidelines may be helpful) a) balance the different types of atoms ________________ b) first, ...
physical change
... Each molecule of a compound contains two or more elements that are chemically combined. ...
... Each molecule of a compound contains two or more elements that are chemically combined. ...
Chemical change is a process that involves recombining atoms and
... one example of chemical change (occurs when a substance or substances react in a chemical reaction to create a different substance or substances) occurring. Other examples of chemical change include dough rising, the changing taste of food cooking on a barbecue, the combustion of fuel in a motor veh ...
... one example of chemical change (occurs when a substance or substances react in a chemical reaction to create a different substance or substances) occurring. Other examples of chemical change include dough rising, the changing taste of food cooking on a barbecue, the combustion of fuel in a motor veh ...
physical and chemical change
... chemical property of iron. In this experiment, you will observe various materials and describe their physical properties. You will then cause some of the materials to undergo changes. Based upon our observations, you will determine whether the changes are physical or chemical changes. Safety First: ...
... chemical property of iron. In this experiment, you will observe various materials and describe their physical properties. You will then cause some of the materials to undergo changes. Based upon our observations, you will determine whether the changes are physical or chemical changes. Safety First: ...
Chemistry - Edgbarrow School
... I can name the main I can represent chemical reactions out simple techniques for changes of state, there are Periodic Table was materials are more reactive elements that make up the using word equations separating mixtures energy changes developed by Mendeleev than others composition of the Earth ...
... I can name the main I can represent chemical reactions out simple techniques for changes of state, there are Periodic Table was materials are more reactive elements that make up the using word equations separating mixtures energy changes developed by Mendeleev than others composition of the Earth ...
Chemical Reactions and Equations
... A Chemical Equation represents a chemical reaction. A complete chemical equation represents the reactants, products, and their physical states symbolically. A chemical equation also indicates a number of atoms of each element involved in a reaction. A chemical equation can be written as balanced or ...
... A Chemical Equation represents a chemical reaction. A complete chemical equation represents the reactants, products, and their physical states symbolically. A chemical equation also indicates a number of atoms of each element involved in a reaction. A chemical equation can be written as balanced or ...
Chemical Reactions and Equations
... compounds are exchanged to give two new compounds. The general equation used to represent double displacement reactions can be written as AB + CD -----> AD + BC Examples of double displacement reactions are Na2SO4 (aq) + BaCl2 (aq) -----> BaSO4 (s) + 2NaCl (aq) “Double Displacement Reactions” have t ...
... compounds are exchanged to give two new compounds. The general equation used to represent double displacement reactions can be written as AB + CD -----> AD + BC Examples of double displacement reactions are Na2SO4 (aq) + BaCl2 (aq) -----> BaSO4 (s) + 2NaCl (aq) “Double Displacement Reactions” have t ...
Document
... 5. Process Chemical Verification In IMDS, only the data of chemicals which are present in the final product should be entered. Therefore, process chemicals used during the manufacturing process are not to be entered and the system should prevent the user from adding those chemicals. There will be a ...
... 5. Process Chemical Verification In IMDS, only the data of chemicals which are present in the final product should be entered. Therefore, process chemicals used during the manufacturing process are not to be entered and the system should prevent the user from adding those chemicals. There will be a ...
Chemical industry

The chemical industry comprises the companies that produce industrial chemicals. Central to the modern world economy, it converts raw materials (oil, natural gas, air, water, metals, and minerals) into more than 70,000 different products.