Amino Acid Starter Kit in Brief
... Notice that some of the side chains have a YELLOW band around the bottom. These side chains are hydrophobic and DO NOT LIKE water. Notice that some of the side chains have a WHITE band around the bottom. These side chains are hydrophilic and DO LIKE water. Notice that some side chains have a RED ban ...
... Notice that some of the side chains have a YELLOW band around the bottom. These side chains are hydrophobic and DO NOT LIKE water. Notice that some of the side chains have a WHITE band around the bottom. These side chains are hydrophilic and DO LIKE water. Notice that some side chains have a RED ban ...
Influenza A H3N2 (A/Perth/16/2009) Hemagglutinin / HA
... Protein Construction: A DNA sequence encoding the Influenza A virus (A/Perth/16/2009 (H3N2)) hemagglutinin (ACS71642.1) (Met1-Lys519, with a mutation Tyr 108 Phe), was expressed with a polyhistidine tag at the C-terminus. ...
... Protein Construction: A DNA sequence encoding the Influenza A virus (A/Perth/16/2009 (H3N2)) hemagglutinin (ACS71642.1) (Met1-Lys519, with a mutation Tyr 108 Phe), was expressed with a polyhistidine tag at the C-terminus. ...
Study Guide Test 3
... 2. Be able to define the following: essential amino acid, non-essential amino acid, branched-chain amino acid and glucogenic amino acid. Know the specific essential amino acids and branched-chain amino acids? 3. Be able to describe the basics of protein metabolism from digestion of protein to protei ...
... 2. Be able to define the following: essential amino acid, non-essential amino acid, branched-chain amino acid and glucogenic amino acid. Know the specific essential amino acids and branched-chain amino acids? 3. Be able to describe the basics of protein metabolism from digestion of protein to protei ...
04b AP Bio The Structure and Function of Proteins and Nucleic
... • At first, all we have is a string of AA’s bound with peptide bonds. • Once the string of AA’s interacts with itself and its environment (often aqueous), then we have a functional protein that consists of one or more polypeptides precisely twisted, folded, and coiled into a unique shape • The seque ...
... • At first, all we have is a string of AA’s bound with peptide bonds. • Once the string of AA’s interacts with itself and its environment (often aqueous), then we have a functional protein that consists of one or more polypeptides precisely twisted, folded, and coiled into a unique shape • The seque ...
The Structure and Function of Macromolecules
... • At first, all we have is a string of AA’s bound with peptide bonds. • Once the string of AA’s interacts with itself and its environment (often aqueous), then we have a functional protein that consists of one or more polypeptides precisely twisted, folded, and coiled into a unique shape • The seque ...
... • At first, all we have is a string of AA’s bound with peptide bonds. • Once the string of AA’s interacts with itself and its environment (often aqueous), then we have a functional protein that consists of one or more polypeptides precisely twisted, folded, and coiled into a unique shape • The seque ...
04b AP Bio The Structure and Function of Proteins and Nucleic
... • At first, all we have is a string of AA’s bound with peptide bonds. • Once the string of AA’s interacts with itself and its environment (often aqueous), then we have a functional protein that consists of one or more polypeptides precisely twisted, folded, and coiled into a unique shape • The seque ...
... • At first, all we have is a string of AA’s bound with peptide bonds. • Once the string of AA’s interacts with itself and its environment (often aqueous), then we have a functional protein that consists of one or more polypeptides precisely twisted, folded, and coiled into a unique shape • The seque ...
Datasheet for Prestained Protein Marker, Broad Range (7
... be boiled upon receipt and aliquotted into singleuse tubes. Store at –20°C. Suggested Protocol for Loading a Sample (2): 1. Mix protein marker. Bring the desired amount of the Prestained Protein Marker over to a separate tube. For blotting: use 6 µl for mini-gels and 12 µl for full length gels. For ...
... be boiled upon receipt and aliquotted into singleuse tubes. Store at –20°C. Suggested Protocol for Loading a Sample (2): 1. Mix protein marker. Bring the desired amount of the Prestained Protein Marker over to a separate tube. For blotting: use 6 µl for mini-gels and 12 µl for full length gels. For ...
Biochemistry Test Review (Vocabulary on the back page
... contain many carbon-hydrogen bonds that also store energy. However, that energy must be transferred to ATP (adenosine triphosphate) to be usable by the cell. B2.2 Organic Molecules There are four major categories of organic molecules that make up living systems: carbohydrates, fats, proteins, and nu ...
... contain many carbon-hydrogen bonds that also store energy. However, that energy must be transferred to ATP (adenosine triphosphate) to be usable by the cell. B2.2 Organic Molecules There are four major categories of organic molecules that make up living systems: carbohydrates, fats, proteins, and nu ...
Assaying
... Highly susceptible to contamination by buffers, biological materials and salts Protein amino acid composition is extremely important, thus the choice of a standard is very difficult, especially for purified proteins Absorbance is heavily influence by pH and ionic strength of the solution. This is of ...
... Highly susceptible to contamination by buffers, biological materials and salts Protein amino acid composition is extremely important, thus the choice of a standard is very difficult, especially for purified proteins Absorbance is heavily influence by pH and ionic strength of the solution. This is of ...
Carbohydrates
... Protein carries out most ALL functions within cell other than genetic storage of information Hemoglobin ...
... Protein carries out most ALL functions within cell other than genetic storage of information Hemoglobin ...
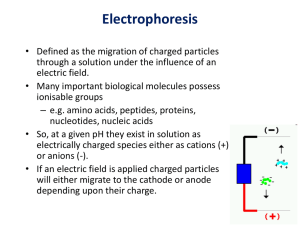
Electrophoresis HCC 2013 BMS2 intro
... • Defined as the migration of charged particles through a solution under the influence of an electric field. • Many important biological molecules possess ionisable groups – e.g. amino acids, peptides, proteins, nucleotides, nucleic acids • So, at a given pH they exist in solution as electrically ch ...
... • Defined as the migration of charged particles through a solution under the influence of an electric field. • Many important biological molecules possess ionisable groups – e.g. amino acids, peptides, proteins, nucleotides, nucleic acids • So, at a given pH they exist in solution as electrically ch ...
Supplementary Information
... regulated by the ubiquitin-proteasome pathway. Key regulator of adherens junction integrity and differentiation ...
... regulated by the ubiquitin-proteasome pathway. Key regulator of adherens junction integrity and differentiation ...
1st Prize: Alex Davison
... cell membrane, inhibiting critical intracellular proteins from functioning and disturbing proteostatic mechanisms such as chaperone molecules and proteasome complexes30. It is also possible that micelle-like oligomers distort transmembrane ion channels, increasing membrane permeability which leads t ...
... cell membrane, inhibiting critical intracellular proteins from functioning and disturbing proteostatic mechanisms such as chaperone molecules and proteasome complexes30. It is also possible that micelle-like oligomers distort transmembrane ion channels, increasing membrane permeability which leads t ...
REVIEW A STRUCTURAL APPROACH TO G
... Interaction with β γ-subunits: The β-subunit shows a propeller structure composed of seven motifs, each comprising fourstranded antiparallel sheets. Each of the seven motifs is called a β-blade. Contact region of αsubunit is opposite to where G γ subunit binds; so there is no contact between α- and ...
... Interaction with β γ-subunits: The β-subunit shows a propeller structure composed of seven motifs, each comprising fourstranded antiparallel sheets. Each of the seven motifs is called a β-blade. Contact region of αsubunit is opposite to where G γ subunit binds; so there is no contact between α- and ...
probing protein structure and interactions using functionalized
... the triplet state. Radicals were observed as lysozyme quenching products. The rate constants for the reaction shown in eq 1 were determined from the bimolecular quenching plots shown in Figure 3. The rate constants and radical (cage escape) yields are summarized in Table 1 (ref. 2). ...
... the triplet state. Radicals were observed as lysozyme quenching products. The rate constants for the reaction shown in eq 1 were determined from the bimolecular quenching plots shown in Figure 3. The rate constants and radical (cage escape) yields are summarized in Table 1 (ref. 2). ...
Detection of α-Synuclein in human plasma and its
... 2. Detection of possible interaction of α-syn with these various lipoprotein fractions from plasma by carrying out immunoblotting using Syn1 antibody. 3. Immunoprecipitation of α-syn from plasma by using an anti-α-syn antibody to capture the entire protein/lipid complex and then determine which apop ...
... 2. Detection of possible interaction of α-syn with these various lipoprotein fractions from plasma by carrying out immunoblotting using Syn1 antibody. 3. Immunoprecipitation of α-syn from plasma by using an anti-α-syn antibody to capture the entire protein/lipid complex and then determine which apop ...
Introduction Document
... biology (the "Graal").Values of all pairs of angles φ (between the Cα atom and the N atom) and ψ (between the Cα atom and the other C atom) for the different amino acids would give exact structure. Very difficult problem. The three dimensional form of a protein is related to its function. A folded p ...
... biology (the "Graal").Values of all pairs of angles φ (between the Cα atom and the N atom) and ψ (between the Cα atom and the other C atom) for the different amino acids would give exact structure. Very difficult problem. The three dimensional form of a protein is related to its function. A folded p ...
Protein Synthesis Molecule by Molecule
... transcription occurred in bursts, with a geometrical distribution of burst sizes - a very similar behavior to that observed by Yu et al. [11] for protein production. This similarity immediately leads to a possible alternative interpretation of the results by Yu et al. [11]: that the observed charact ...
... transcription occurred in bursts, with a geometrical distribution of burst sizes - a very similar behavior to that observed by Yu et al. [11] for protein production. This similarity immediately leads to a possible alternative interpretation of the results by Yu et al. [11]: that the observed charact ...
Unit 1 Objectives 2015
... 2. Explain the uses of carbon, hydrogen, oxygen, nitrogen, phosphorous and sulfur in biological systems. 3. Diagram the exchange of matter between organisms and the environment. 4. What function does nitrogen serve in proteins? In nucleic acids? 5. What function does phosphorus serve in nucleic acid ...
... 2. Explain the uses of carbon, hydrogen, oxygen, nitrogen, phosphorous and sulfur in biological systems. 3. Diagram the exchange of matter between organisms and the environment. 4. What function does nitrogen serve in proteins? In nucleic acids? 5. What function does phosphorus serve in nucleic acid ...
Ten novel interaction partners for the histone H2A protein
... fragments fused to the N-terminal half of ubiquitin was transformed into an S. cerevisiae strain carrying the bait protein H2A (encoded by the gene HTA1) fused to the C-terminal half of ubiquitin. Protein interactions with the bait protein resulted in a native-like ubiquitin being formed, allowing f ...
... fragments fused to the N-terminal half of ubiquitin was transformed into an S. cerevisiae strain carrying the bait protein H2A (encoded by the gene HTA1) fused to the C-terminal half of ubiquitin. Protein interactions with the bait protein resulted in a native-like ubiquitin being formed, allowing f ...
MS Word File
... Tertiary structures are larger folding events that are stabilized by interactions between R groups Quaternary structure is the interaction of multiple polypeptides within one active proteins Primary Structure ...
... Tertiary structures are larger folding events that are stabilized by interactions between R groups Quaternary structure is the interaction of multiple polypeptides within one active proteins Primary Structure ...
Proteins - Clayton State University
... • Therefore, the term applies specifically to multimeric proteins • Some proteins consist of multiple identical subunits; others, like hemoglobin, contain two or more types of polypeptides ...
... • Therefore, the term applies specifically to multimeric proteins • Some proteins consist of multiple identical subunits; others, like hemoglobin, contain two or more types of polypeptides ...
Modes of Macromolecular Classification
... But how are we to understand tertiary structure? We might abstract away from the peptide bonds (the links between individual amino acids) and think of a protein’s three-dimensional structure as simply the relative location of individual amino acids (in the manner we think of a crystalline structure ...
... But how are we to understand tertiary structure? We might abstract away from the peptide bonds (the links between individual amino acids) and think of a protein’s three-dimensional structure as simply the relative location of individual amino acids (in the manner we think of a crystalline structure ...
Protein–protein interaction

Protein–protein interactions (PPIs) refer to physical contacts established between two or more proteins as a result of biochemical events and/or electrostatic forces.In fact, proteins are vital macromolecules, at both cellular and systemic levels, but they rarely act alone. Diverse essential molecular processes within a cell are carried out by molecular machines that are built from a large number of protein components organized by their PPIs. Indeed, these interactions are at the core of the entire interactomics system of any living cell and so, unsurprisingly, aberrant PPIs are on the basis of multiple diseases, such as Creutzfeld-Jacob, Alzheimer's disease, and cancer.PPIs have been studied from different perspectives: biochemistry, quantum chemistry, molecular dynamics, signal transduction, among others. All this information enables the creation of large protein interaction networks – similar to metabolic or genetic/epigenetic networks – that empower the current knowledge on biochemical cascades and disease pathogenesis, as well as provide putative new therapeutic targets.