p Block Elements General Configuration: ns2 np1
... Nitrogen differs from the rest of the members of this group due to its smaller size, high electro negativity, high ionization enthalpy and non-availability of d-orbitals. Nitrogen can form pπ-pπ multiple bond. Nitrogen exists as diatomic molecule with a triple bond. Heavier elements do not form pπ-p ...
... Nitrogen differs from the rest of the members of this group due to its smaller size, high electro negativity, high ionization enthalpy and non-availability of d-orbitals. Nitrogen can form pπ-pπ multiple bond. Nitrogen exists as diatomic molecule with a triple bond. Heavier elements do not form pπ-p ...
Next > Mendeleev and Meyer
... which they lose when they form bonds with other atoms. Some transition elements can lose electrons in their next-to-outermost level Many transition metals combine chemically with oxygen to form compounds called oxides. ...
... which they lose when they form bonds with other atoms. Some transition elements can lose electrons in their next-to-outermost level Many transition metals combine chemically with oxygen to form compounds called oxides. ...
Attachment: Click to download
... Atoms are so small, it is difficult to discuss how much they weigh in grams Use atomic mass units. an atomic mass unit (amu) is one twelfth the mass of a carbon-12 atom This gives us a basis for comparison The decimal numbers on the table are atomic masses in amu ...
... Atoms are so small, it is difficult to discuss how much they weigh in grams Use atomic mass units. an atomic mass unit (amu) is one twelfth the mass of a carbon-12 atom This gives us a basis for comparison The decimal numbers on the table are atomic masses in amu ...
Introduction To The Periodic Table Of The Elements
... React rapidly when exposed to air and water ...
... React rapidly when exposed to air and water ...
Review for Physical Science Test #2
... Review for Compounds, Reactions, Acids & Bases Test Compounds 1. Compounds are made of ______________________ of elements that are _______________________________ together. 2. What are two ways that atoms can be bonded together? (Hint: both have to do with electrons.) ...
... Review for Compounds, Reactions, Acids & Bases Test Compounds 1. Compounds are made of ______________________ of elements that are _______________________________ together. 2. What are two ways that atoms can be bonded together? (Hint: both have to do with electrons.) ...
Chemistry Notes
... The mole interpretation is the more practical interpretation because we can not see single molecules and atoms for everyday work. So how many moles of carbon is in C12H22O11 (table sugar)? Write the answer in your notes. ...
... The mole interpretation is the more practical interpretation because we can not see single molecules and atoms for everyday work. So how many moles of carbon is in C12H22O11 (table sugar)? Write the answer in your notes. ...
Chapter 6 Study Guide
... a. period 3, Group IIIA _____________________________________________ b. period 1, Group VIIIA ____________________________________________ c. period 4, Group IIA ______________________________________________ d. period 6, Group VA ______________________________________________ 7. How many Valence e ...
... a. period 3, Group IIIA _____________________________________________ b. period 1, Group VIIIA ____________________________________________ c. period 4, Group IIA ______________________________________________ d. period 6, Group VA ______________________________________________ 7. How many Valence e ...
Review of Periodic Trends
... upper left hand corner of the periodic table lower left hand corner of the periodic table lower right hand corner of the periodic table upper right hand corner of the periodic table ...
... upper left hand corner of the periodic table lower left hand corner of the periodic table lower right hand corner of the periodic table upper right hand corner of the periodic table ...
2016 - Specimen Paper 4 - Cambridge International Examinations
... number of moles of HCl used = ………………………………………………...…..……. number of moles of CoCl2 formed = ……………………………………………..….…… number of moles of CoCl2.6H2O formed = ………………………………………..…..… mass of one mole of CoCl2.6H2O = 238 g maximum yield of CoCl2.6H2O = …………………………………………………..…..… g to show that cobalt(II) ca ...
... number of moles of HCl used = ………………………………………………...…..……. number of moles of CoCl2 formed = ……………………………………………..….…… number of moles of CoCl2.6H2O formed = ………………………………………..…..… mass of one mole of CoCl2.6H2O = 238 g maximum yield of CoCl2.6H2O = …………………………………………………..…..… g to show that cobalt(II) ca ...
File - Mr. Walsh`s AP Chemistry
... 8) Based on the information in the table below, what is the most likely charge of element X? _________ ...
... 8) Based on the information in the table below, what is the most likely charge of element X? _________ ...
Periodic Table
... Sulfur was one of the first elements to be discovered because it occurs in large deposits in the Earth. It is used to make rubber and sulfuric acid. ...
... Sulfur was one of the first elements to be discovered because it occurs in large deposits in the Earth. It is used to make rubber and sulfuric acid. ...
Compounds have different properties from the elements that make
... substances are compounds. A compound is a substance made of atoms of two or more different elements. Just as the 26 letters in the alphabet can form thousands of words, the elements in the periodic table can form millions of compounds. The atoms of different elements are held together in compounds b ...
... substances are compounds. A compound is a substance made of atoms of two or more different elements. Just as the 26 letters in the alphabet can form thousands of words, the elements in the periodic table can form millions of compounds. The atoms of different elements are held together in compounds b ...
Intermediate 1 Chemistry - Deans Community High School
... 3) Helium is lighter then air and is used in balloons and airships (as well as for talking in a silly voice) 4) Argon is used in light bulbs (because it is so unreactive) and argon , krypton and neon are used in fancy lights ...
... 3) Helium is lighter then air and is used in balloons and airships (as well as for talking in a silly voice) 4) Argon is used in light bulbs (because it is so unreactive) and argon , krypton and neon are used in fancy lights ...
The periodic table is a map of the elements.
... wide range of properties. • Nonmetals – to the right side of the periodic table and have properties the opposite of metals • Many are gases at room temp, and one – bromine – is a liquid • Solid nonmetals – often have dull surfaces and cannot be shaped by hammering or drawn into wires • Generally poo ...
... wide range of properties. • Nonmetals – to the right side of the periodic table and have properties the opposite of metals • Many are gases at room temp, and one – bromine – is a liquid • Solid nonmetals – often have dull surfaces and cannot be shaped by hammering or drawn into wires • Generally poo ...
Unit 9 Chemical Equations and Reactions Balancing Equations Notes
... What is a Chemical Equation? A _________________________ is a written representation of the process that occurs in a chemical reaction. A chemical equation is written with the _______________ (starting materials) on the left side of an arrow and the ___________________ (resulting substance) of the c ...
... What is a Chemical Equation? A _________________________ is a written representation of the process that occurs in a chemical reaction. A chemical equation is written with the _______________ (starting materials) on the left side of an arrow and the ___________________ (resulting substance) of the c ...
2007 - SolPass
... electronic or mechanical, including photocopying or recording, or by any information storage or retrieval system, without written permission from the copyright owner. Commonwealth of Virginia public school educators may reproduce any portion of these released tests for non-commercial educational pur ...
... electronic or mechanical, including photocopying or recording, or by any information storage or retrieval system, without written permission from the copyright owner. Commonwealth of Virginia public school educators may reproduce any portion of these released tests for non-commercial educational pur ...
Worksheet 3.2 - contentextra
... and Si4+ are isoelectronic and have the same electron arrangement 2, 8. The decrease in ionic radius is due to the increase in nuclear charge with atomic number across the period, which increases the attraction between the nucleus and the outer electrons. ...
... and Si4+ are isoelectronic and have the same electron arrangement 2, 8. The decrease in ionic radius is due to the increase in nuclear charge with atomic number across the period, which increases the attraction between the nucleus and the outer electrons. ...
end of year review
... a. can be in any ratio. c. Retain their original identifying properties. b. can be separated easily. d. Chemically unite to form one substance. _____ 3. The graph below compares three states of a substance. ...
... a. can be in any ratio. c. Retain their original identifying properties. b. can be separated easily. d. Chemically unite to form one substance. _____ 3. The graph below compares three states of a substance. ...
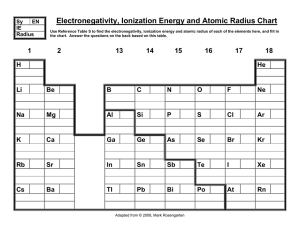
Electronegativity, Ionization Energy and Atomic Radius Chart
... - Transition metals can be very reactive (like Zn) or slightly reactive (like Au). - Transition metals lose electrons from their valence shell and the shell beneath it. - Transition metals can form more than one ion charge (Cr can form a charge of +2, +3 or +6) - Transition metal compounds tend to b ...
... - Transition metals can be very reactive (like Zn) or slightly reactive (like Au). - Transition metals lose electrons from their valence shell and the shell beneath it. - Transition metals can form more than one ion charge (Cr can form a charge of +2, +3 or +6) - Transition metal compounds tend to b ...
MIDTERM REVIEW UNIT 1: Mass/Measurement
... 11. In a reaction between lead (II) nitrate and copper (II) bromide, do the following: a) write the formulas for the reactants and the products and balance the equation b) If 0.67 moles of copper (II) ...
... 11. In a reaction between lead (II) nitrate and copper (II) bromide, do the following: a) write the formulas for the reactants and the products and balance the equation b) If 0.67 moles of copper (II) ...
Second Semester Final Review Guide
... grams of iron, 210.385 grams of oxygen. The compounds molar mass is 159.697 grams/ mole. a. What percentage of the compound is aluminum?____________ b. What percentage of the compound is oxygen?_____________ c. What percentage of the compound is sulfur?_____________ d. What is the empirical formula ...
... grams of iron, 210.385 grams of oxygen. The compounds molar mass is 159.697 grams/ mole. a. What percentage of the compound is aluminum?____________ b. What percentage of the compound is oxygen?_____________ c. What percentage of the compound is sulfur?_____________ d. What is the empirical formula ...
Unit 8 Packet - Page 1 of 18 Honors Chemistry
... When writing equations you must satisfy the law of conservation matter (matter can not be created or destroyed) Therefore we must have the same type and number of each atom on each side of the equation. BALANCING EQUATIONS: 4 steps: 1. Start with a word equation 2. Convert to a formula equation (don ...
... When writing equations you must satisfy the law of conservation matter (matter can not be created or destroyed) Therefore we must have the same type and number of each atom on each side of the equation. BALANCING EQUATIONS: 4 steps: 1. Start with a word equation 2. Convert to a formula equation (don ...