Experimental Approaches to Protein–Protein Interactions
... The interactome (i.e. the set of interactions between all proteins in the cell) of the yeast Saccharomyces cerevisiae has been described by two independent groups, in both cases using TAP tagging [8,9]. The results are broadly consistent, in that they show that approx. 70% of proteins in the cell ha ...
... The interactome (i.e. the set of interactions between all proteins in the cell) of the yeast Saccharomyces cerevisiae has been described by two independent groups, in both cases using TAP tagging [8,9]. The results are broadly consistent, in that they show that approx. 70% of proteins in the cell ha ...
Diabetes
... Insulin is a hormone. It is made of protein and is involved in the regulation of glucose levels in the blood ...
... Insulin is a hormone. It is made of protein and is involved in the regulation of glucose levels in the blood ...
L9 Protein cross links - e
... It can be attributed to the formation of intermolecular cross-links between proteins, although no evidence has yet been provided. SOX might oxidize the reduced glutathione present in wheat flour. Reduced glutathione could also react with gluten weakening the disulfide bond network of gluten, whi ...
... It can be attributed to the formation of intermolecular cross-links between proteins, although no evidence has yet been provided. SOX might oxidize the reduced glutathione present in wheat flour. Reduced glutathione could also react with gluten weakening the disulfide bond network of gluten, whi ...
Biosynthesis of a Secretory Protein
... Ribosome makes the polypeptide in the cytoplasm Polypeptide goes through a protein import channel in the RER. Within the RER, the polypeptide is cleaved, sugar added, and polypeptide folds to take a specific shape. Soluble proteins are transported in a transport vesicle to the Golgi Body by exocytos ...
... Ribosome makes the polypeptide in the cytoplasm Polypeptide goes through a protein import channel in the RER. Within the RER, the polypeptide is cleaved, sugar added, and polypeptide folds to take a specific shape. Soluble proteins are transported in a transport vesicle to the Golgi Body by exocytos ...
Sample Preparation Guidelines for 2
... that interfere with the labeling of the protein sample with CyDye Fluor minimal dyes. These reagents include thiols used as reducing agents and polyamines used as IPG buffers (ampholytes). Samples must also be free of ionic contaminants that hamper effective isoelectric focusing of the proteins duri ...
... that interfere with the labeling of the protein sample with CyDye Fluor minimal dyes. These reagents include thiols used as reducing agents and polyamines used as IPG buffers (ampholytes). Samples must also be free of ionic contaminants that hamper effective isoelectric focusing of the proteins duri ...
PATHOPHYSIOLOGY LAB QUESTIONS Laboratory
... phase reactants are normal, haptoglobin is decreased. Serum iron concentration is high, total iron binding capacity (TIBC) is decreased, total serum LDH activity is elevated. What is the most likely diagnosis? ...
... phase reactants are normal, haptoglobin is decreased. Serum iron concentration is high, total iron binding capacity (TIBC) is decreased, total serum LDH activity is elevated. What is the most likely diagnosis? ...
a version - SEA
... upstream of a transmembrane helix, indicating that these motifs may correspond to conserved, functional regions in holins. There is no apparent conservation of the holin family in closely related bacteriophage clusters (Splitstree), indicating that holin proteins are highly variable and not strongly ...
... upstream of a transmembrane helix, indicating that these motifs may correspond to conserved, functional regions in holins. There is no apparent conservation of the holin family in closely related bacteriophage clusters (Splitstree), indicating that holin proteins are highly variable and not strongly ...
Assignments 3 Problem 1 Below is the protein melting data for a pair
... wild-type protein. b) Determine your signal for the fully folded and the fully unfolded form (both mutant and WT). c) Plot the melting curves as the fraction of folded protein vs. temp. d) What are the melting temperatures for the two proteins? e) Pick the appropriate plots to determine ΔH and ΔS of ...
... wild-type protein. b) Determine your signal for the fully folded and the fully unfolded form (both mutant and WT). c) Plot the melting curves as the fraction of folded protein vs. temp. d) What are the melting temperatures for the two proteins? e) Pick the appropriate plots to determine ΔH and ΔS of ...
Slides - University of Minnesota
... • Shown correlation with structure, sequence and phylogeny • Explored structure-function relation via FDUG (functional domain universe graph) • Indicated examples of divergent and convergent evolution ...
... • Shown correlation with structure, sequence and phylogeny • Explored structure-function relation via FDUG (functional domain universe graph) • Indicated examples of divergent and convergent evolution ...
Chem*3560 Lecture 19: Review of regulation
... hemoglobin). The T/R terminology is now applied to all enzymes showing sigmoidal kinetics. The steep rise in the sigmoidal curve occurs as the protein starts to switch states. Individual molecules of the enzyme do not go through intermediate stages. Rather, the intermediate stage represents a certai ...
... hemoglobin). The T/R terminology is now applied to all enzymes showing sigmoidal kinetics. The steep rise in the sigmoidal curve occurs as the protein starts to switch states. Individual molecules of the enzyme do not go through intermediate stages. Rather, the intermediate stage represents a certai ...
Monoclonal Anti-c-Myc-Biotin, clone 9E10 (B7554)
... myeloma cells and splenocytes from BALB/c mice immunized with a synthetic peptide corresponding to c-myc residues 408-439 of the human p62 protein ...
... myeloma cells and splenocytes from BALB/c mice immunized with a synthetic peptide corresponding to c-myc residues 408-439 of the human p62 protein ...
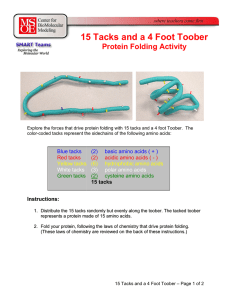
15 Tacks and a 4 Foot Toober
... • And pairing green tacks that form disulfide bonds, • And keeping all of the polar white tacks on the surface of the protein. After everyone has folded their toober as best they can, the teacher can point out: • Every toober had a different random sequence of tacks (amino acids) and therefore each ...
... • And pairing green tacks that form disulfide bonds, • And keeping all of the polar white tacks on the surface of the protein. After everyone has folded their toober as best they can, the teacher can point out: • Every toober had a different random sequence of tacks (amino acids) and therefore each ...
This document present*s EMS, LLC*s standard operating
... for Restricted Use Protein Products (RUPP) to enhance consumer confidence in the feed and food supply. This plan incorporates the FDA's inspection program for compliance with Title 21, CFR § 589.2000, Substances Prohibited in Ruminant Feed. Under the program independent certifying Agents visit facil ...
... for Restricted Use Protein Products (RUPP) to enhance consumer confidence in the feed and food supply. This plan incorporates the FDA's inspection program for compliance with Title 21, CFR § 589.2000, Substances Prohibited in Ruminant Feed. Under the program independent certifying Agents visit facil ...
Poster
... the ubiquitin activating enzyme (E1), the ubiquitin conjugating enzyme (E2), and the ubiquitin ligase (E3). Ubiquitination begins with ubiquitin being activated by and attaching to E1. E1 transfers ubiquitin to E2. Then E2 delivers ubiquitin to the unwanted protein, either directly or through E3. Fi ...
... the ubiquitin activating enzyme (E1), the ubiquitin conjugating enzyme (E2), and the ubiquitin ligase (E3). Ubiquitination begins with ubiquitin being activated by and attaching to E1. E1 transfers ubiquitin to E2. Then E2 delivers ubiquitin to the unwanted protein, either directly or through E3. Fi ...
Choose the best answer for the following questions
... (C). A protein only folds correctly when present in its normal cellular environment. 7. During attachment of an amino acid to its tRNA, how is ATP required? (A). The free energy of hydrolysis of ATP is used to provide the energy necessary to link the free amino acid to the tRNA. (B). The ATP forms a ...
... (C). A protein only folds correctly when present in its normal cellular environment. 7. During attachment of an amino acid to its tRNA, how is ATP required? (A). The free energy of hydrolysis of ATP is used to provide the energy necessary to link the free amino acid to the tRNA. (B). The ATP forms a ...
Phase behaviour and transitions of peptides and proteins
... The intrinsic property of proteins to form structural motifs such as α helices and β sheets leads to a complex phase behavior in which proteins can assemble into various types of aggregates including crystals, liquidlike phases of unfolded or natively folded proteins, and amyloid fibrils. In this wo ...
... The intrinsic property of proteins to form structural motifs such as α helices and β sheets leads to a complex phase behavior in which proteins can assemble into various types of aggregates including crystals, liquidlike phases of unfolded or natively folded proteins, and amyloid fibrils. In this wo ...
TD11 Identification of in vivo substrates of GroEL Nature 1999, 402
... 1) punch out single hot spot from gel (can increase the amount of protein at spot by co-running w/ cold crude bacterial lysate->there is more of the same, unlabeled protein at that spot for analysis) 2) extract protein from gel 3) complete digest with trypsin protease (cleaves C-terminal to Lys and ...
... 1) punch out single hot spot from gel (can increase the amount of protein at spot by co-running w/ cold crude bacterial lysate->there is more of the same, unlabeled protein at that spot for analysis) 2) extract protein from gel 3) complete digest with trypsin protease (cleaves C-terminal to Lys and ...
A Figure S7. A. Standard curve of actin quantification using silver
... Figure S7. A. Standard curve of actin quantification using silver staining. Actin standards were prepared by serial dilution and separated using SDS gel electrophoresis. Silver staining was carried out and band quantification was accomplished using the BioRad QuantityOne software. Linear regression ...
... Figure S7. A. Standard curve of actin quantification using silver staining. Actin standards were prepared by serial dilution and separated using SDS gel electrophoresis. Silver staining was carried out and band quantification was accomplished using the BioRad QuantityOne software. Linear regression ...
HomeworkCh_15,16Answers
... 1. Plants do not eat but do require CO2, H2O, and sunlight to live. How is this different from animals? What system do plants have to allow for their simple needs? Animals require nutrients such as carbohydrates, proteins and lipids to survive. Photosynthesis. 2. What substances constitute the macro ...
... 1. Plants do not eat but do require CO2, H2O, and sunlight to live. How is this different from animals? What system do plants have to allow for their simple needs? Animals require nutrients such as carbohydrates, proteins and lipids to survive. Photosynthesis. 2. What substances constitute the macro ...
Culinary Chemistry: A Campus Cuisine Cookoff Michele McMullen R.D. Dr. Matt Queen
... Denaturing proteins: Just apply heat ...
... Denaturing proteins: Just apply heat ...
2,3-BPG and the O 2
... contrary to Hb, myoglobin (Mb) is a monomeric O2-storage molecule, evolutionary related to Hb, a very similar structure to Hb (globin fold) Hb can use 90% of its full O2-binding potential, while Mb can use only 7% in Hb there is a so-called cooperativity, so O2-binding to one chain potentiates O2-bi ...
... contrary to Hb, myoglobin (Mb) is a monomeric O2-storage molecule, evolutionary related to Hb, a very similar structure to Hb (globin fold) Hb can use 90% of its full O2-binding potential, while Mb can use only 7% in Hb there is a so-called cooperativity, so O2-binding to one chain potentiates O2-bi ...
AMIDO BLACK PROTEIN ASSAY
... Subject: Micro-protein assay This micro-protein assay is not as accurate as other methods for assaying protein concentration, but it has the distinct advantage of requiring only trace amounts (less than 1ug) of your valuable protein. In many cases the accuracy (within a factor of 2) of the assay is ...
... Subject: Micro-protein assay This micro-protein assay is not as accurate as other methods for assaying protein concentration, but it has the distinct advantage of requiring only trace amounts (less than 1ug) of your valuable protein. In many cases the accuracy (within a factor of 2) of the assay is ...
Protein folding

Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil.Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acids. This polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the native state. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma). Experiments beginning in the 1980s indicate the codon for an amino acid can also influence protein structure.The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded, so that protein dynamics is important. Failure to fold into native structure generally produces inactive proteins, but in some instances misfolded proteins have modified or toxic functionality. Several neurodegenerative and other diseases are believed to result from the accumulation of amyloid fibrils formed by misfolded proteins. Many allergies are caused by incorrect folding of some proteins, because the immune system does not produce antibodies for certain protein structures.