Chapter 8 - Inorganic carbon chemistry
... As well as giving rise to beautiful and varied countryside, limestone is a very useful raw material. Limestone is composed of calcium carbonate (CaCO3) in the form of the mineral calcite (Figure 8.2). Chalk and marble are also made of calcite which is the second most abundant mineral in the Earth's ...
... As well as giving rise to beautiful and varied countryside, limestone is a very useful raw material. Limestone is composed of calcium carbonate (CaCO3) in the form of the mineral calcite (Figure 8.2). Chalk and marble are also made of calcite which is the second most abundant mineral in the Earth's ...
03CAM 2011 - AP Bio Take 5
... PHYSICALLY separate carbon fixation from Calvin cycle different cells to fix carbon vs. where Calvin cycle occurs store carbon in 4C compounds different enzyme to capture CO2 (fix carbon) ...
... PHYSICALLY separate carbon fixation from Calvin cycle different cells to fix carbon vs. where Calvin cycle occurs store carbon in 4C compounds different enzyme to capture CO2 (fix carbon) ...
Alice and Lewis Carroll
... • Before 1951, only relative configurations could be known. • Sugars and amino acids with same relative configuration as (+)-glyceraldehyde were assigned D and same as (-)glyceraldehyde were assigned L. • With X-ray crystallography, now know absolute configurations: D is (R) and L is (S). • No relat ...
... • Before 1951, only relative configurations could be known. • Sugars and amino acids with same relative configuration as (+)-glyceraldehyde were assigned D and same as (-)glyceraldehyde were assigned L. • With X-ray crystallography, now know absolute configurations: D is (R) and L is (S). • No relat ...
L20 Medicinal Ch 28.07.2015 Metabolism
... stays away from steric crowdedness as much as possible. ...
... stays away from steric crowdedness as much as possible. ...
Class 11 Class 12 The p- Block Element • Group13 (B to Tl
... http://ncerthelp.com/text.php?ques=1391+P+Block+Elements+Modern+PeriodicTtable+Notes+Download+in+PDF[12/19/2016 11:06:09 AM] ...
... http://ncerthelp.com/text.php?ques=1391+P+Block+Elements+Modern+PeriodicTtable+Notes+Download+in+PDF[12/19/2016 11:06:09 AM] ...
Carbon Chemistry - North Allegheny School District
... shown in Figure 4. The names and the chemical formulas of a few of the smaller saturated hydrocarbons are listed in Table 1. Saturated hydrocarbons are named with an -ane ending. Another name for these hydrocarbons is alkanes. What is a saturated hydrocarbon? ...
... shown in Figure 4. The names and the chemical formulas of a few of the smaller saturated hydrocarbons are listed in Table 1. Saturated hydrocarbons are named with an -ane ending. Another name for these hydrocarbons is alkanes. What is a saturated hydrocarbon? ...
Chapter 4 - Jenkins Independent Schools
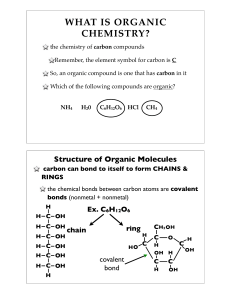
... Earth’s crust contains less than one percent carbon, yet all living things on Earth are made of carbon-containing compounds. Carbon’s ability to bond easily and form compounds is the basis of life on Earth. A carbon atom has four electrons in its outer energy level, so it can form covalent bonds wit ...
... Earth’s crust contains less than one percent carbon, yet all living things on Earth are made of carbon-containing compounds. Carbon’s ability to bond easily and form compounds is the basis of life on Earth. A carbon atom has four electrons in its outer energy level, so it can form covalent bonds wit ...
Chiral Carbon Atoms
... Enantiomers rotate plane polarized light in opposite directions of each other. (+): Rotation to the right (-): Rotation to the left You can not assign absolute configuration based on optical rotation alone. (R)-enantiomers can be (+) or (-) and (S)-enantiomers can be (+) or (-). ...
... Enantiomers rotate plane polarized light in opposite directions of each other. (+): Rotation to the right (-): Rotation to the left You can not assign absolute configuration based on optical rotation alone. (R)-enantiomers can be (+) or (-) and (S)-enantiomers can be (+) or (-). ...
Solutions_C17
... 21. Two oxygen and one carbon atom combine to form carbon dioxide. Complete the following steps to construct a structural diagram for CO2. 21a. Draw the LDS diagrams for oxygen and carbon. A. ...
... 21. Two oxygen and one carbon atom combine to form carbon dioxide. Complete the following steps to construct a structural diagram for CO2. 21a. Draw the LDS diagrams for oxygen and carbon. A. ...
Alkanes Chapter 1.1
... Branched Alkanes • A substituent group is an atom or group of atoms that replaces a hydrogen atom in an organic compound • An alkyl group is a type of substituent group made up of one or more carbon atoms • Branches are named using a three part prefix 1. A number to indicate which carbon on the mai ...
... Branched Alkanes • A substituent group is an atom or group of atoms that replaces a hydrogen atom in an organic compound • An alkyl group is a type of substituent group made up of one or more carbon atoms • Branches are named using a three part prefix 1. A number to indicate which carbon on the mai ...
1. Introduction - UBC ECE - University of British Columbia
... value of the final separation between the amino acid dimers and the nanotube was about 0.25 nm in all cases. Using the examples of the (10,0) tubes, one of the nearest dimers was chosen from each of the ASN and ILE cases for use in the DFT/NEGF simulations. To reduce the size of the simulation space ...
... value of the final separation between the amino acid dimers and the nanotube was about 0.25 nm in all cases. Using the examples of the (10,0) tubes, one of the nearest dimers was chosen from each of the ASN and ILE cases for use in the DFT/NEGF simulations. To reduce the size of the simulation space ...
14 Chapter
... Section 1: Simple Organic Compounds Section 2: Other Organic Compounds Section 3: Biological Compounds ...
... Section 1: Simple Organic Compounds Section 2: Other Organic Compounds Section 3: Biological Compounds ...
9.1-10.5 Organic Chemistry
... Remember Lewis Dot Diagrams from Chem 20?? This means carbon can bond extensively and can bond together to form chains effectively = called Polymerism Carbon covalently bonds by sharing 4 pairs of electrons. These bonds may be single, double or triple, all producing stable compounds Compound ...
... Remember Lewis Dot Diagrams from Chem 20?? This means carbon can bond extensively and can bond together to form chains effectively = called Polymerism Carbon covalently bonds by sharing 4 pairs of electrons. These bonds may be single, double or triple, all producing stable compounds Compound ...
9.1-10.5 Organic Chemistry
... Remember Lewis Dot Diagrams from Chem 20?? This means carbon can bond extensively and can bond together to form chains effectively = called Polymerism Carbon covalently bonds by sharing 4 pairs of electrons. These bonds may be single, double or triple, all producing stable compounds Compound ...
... Remember Lewis Dot Diagrams from Chem 20?? This means carbon can bond extensively and can bond together to form chains effectively = called Polymerism Carbon covalently bonds by sharing 4 pairs of electrons. These bonds may be single, double or triple, all producing stable compounds Compound ...
GRAPHITE
... Graphite structure The crystalline structure of graphite consists of hexagonal rings forming thin parallel plates (graphenes). Each carbon atom is covalently bonded to three other atoms in the plate (the angle between two bonds is 120°). The outermost electron shell of a carbon atom has four valence ...
... Graphite structure The crystalline structure of graphite consists of hexagonal rings forming thin parallel plates (graphenes). Each carbon atom is covalently bonded to three other atoms in the plate (the angle between two bonds is 120°). The outermost electron shell of a carbon atom has four valence ...
Organic and Bio-Molecular Chemistry
... two linkages; nitrogen, boron and aluminum are trivalent, they can establish three linkages with different atoms. Therefore silicon and carbon, the two abundant tetravalent elements, are the most efficient scaffolds to build up tridimensional molecular structures. There is however an important diffe ...
... two linkages; nitrogen, boron and aluminum are trivalent, they can establish three linkages with different atoms. Therefore silicon and carbon, the two abundant tetravalent elements, are the most efficient scaffolds to build up tridimensional molecular structures. There is however an important diffe ...
carbon compounds - Badhan Education
... Carbon compounds large number compounds with hydrogen called hydrocarbons. Carbon can form compounds with hydrogen, oxygen, nitrogen, sulphur etc. Today, million of carbon compounds are known. These compounds are used in our daily life and they have brought lot of changes in our life. They are being ...
... Carbon compounds large number compounds with hydrogen called hydrocarbons. Carbon can form compounds with hydrogen, oxygen, nitrogen, sulphur etc. Today, million of carbon compounds are known. These compounds are used in our daily life and they have brought lot of changes in our life. They are being ...
Nuts,Bolts and Isotopes- Average Atomic Mass Activity
... (for example carbon is composed of carbon atoms). However, not all of the atoms found in that element are the same. For example, carbon contains three different types of atoms (carbon-12, 13 and 14). Each atom has the same number of protons and electrons but differing numbers of neutrons. These are ...
... (for example carbon is composed of carbon atoms). However, not all of the atoms found in that element are the same. For example, carbon contains three different types of atoms (carbon-12, 13 and 14). Each atom has the same number of protons and electrons but differing numbers of neutrons. These are ...
Document
... they are ISOMERS of each other they can only be absorbed into the intestine & blood stream when they are broken down into their monomers (monosaccharides) by enzymes Examples ...
... they are ISOMERS of each other they can only be absorbed into the intestine & blood stream when they are broken down into their monomers (monosaccharides) by enzymes Examples ...
Section 2-3: Carbon Compounds (p. 44-48)
... Not only can carbon atoms bond to H, N, O, P and S ...
... Not only can carbon atoms bond to H, N, O, P and S ...
A summary of amino acid metabolism based on amino acid structure
... Figure 3 Examples of the relationship between amino acid structure and metabolism (a) Tryptophan has at least three hydrocarbon carbons in a row beginning with the f5 carbon (carbon 3) and thus must be converted, at least in part, to acetyl CoA (hydrocarbon carbons are labeled a, b, c, d, e, f, and ...
... Figure 3 Examples of the relationship between amino acid structure and metabolism (a) Tryptophan has at least three hydrocarbon carbons in a row beginning with the f5 carbon (carbon 3) and thus must be converted, at least in part, to acetyl CoA (hydrocarbon carbons are labeled a, b, c, d, e, f, and ...
Lecture 8. Biogeochemical Cycles
... emerged because nitrogen was a limiting element for microbial growth. Although molecular nitrogen was abundant in the atmosphere, microbial cells could not directly utilize nitrogen as N2 gas. Cells require organic nitrogen compounds or reduced inorganic forms of nitrogen for growth. Therefore, unde ...
... emerged because nitrogen was a limiting element for microbial growth. Although molecular nitrogen was abundant in the atmosphere, microbial cells could not directly utilize nitrogen as N2 gas. Cells require organic nitrogen compounds or reduced inorganic forms of nitrogen for growth. Therefore, unde ...
carbohydrates
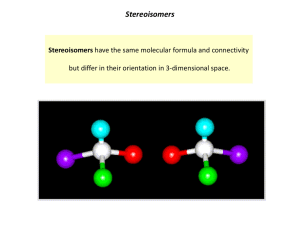
... Stereoisomers that are not mirror images; may have same D-‐ or L-‐ configura=on (or not). Configura=onal: Anomers-‐ Stereoisomers that differ in configura=on at the anomeric carbon (formerly the carbonyl C). ...
... Stereoisomers that are not mirror images; may have same D-‐ or L-‐ configura=on (or not). Configura=onal: Anomers-‐ Stereoisomers that differ in configura=on at the anomeric carbon (formerly the carbonyl C). ...
Silicon Carbide Coating for Carbon Materials Produced by a
... renewed. A failure face of such a sample is shown in figure 3. The weight increases are 14.9, 9.3, and 7.4 mg for the first, second and third cementation, respectively. The corresponding S i c thicknesses are 80,60, and 50 pm. The 14.9 mg and 80 pm values for the first cementation agree perfectly wi ...
... renewed. A failure face of such a sample is shown in figure 3. The weight increases are 14.9, 9.3, and 7.4 mg for the first, second and third cementation, respectively. The corresponding S i c thicknesses are 80,60, and 50 pm. The 14.9 mg and 80 pm values for the first cementation agree perfectly wi ...
Organic Chemistry 2014 finalzzz
... The numbering for the carbon atoms begins with the double bond; the carbons of the double bond are carbons 1 and 2; lowest numbers possible ...
... The numbering for the carbon atoms begins with the double bond; the carbons of the double bond are carbons 1 and 2; lowest numbers possible ...
Carbon

Carbon (from Latin: carbo ""coal"") is a chemical element with symbol C and atomic number 6. On the Periodic table, it is the first (row 2) of six elements in column (group) 14, which have in common the composition of their outer electron shell. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. There are three naturally occurring isotopes, with 12C and 13C being stable, while 14C is radioactive, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity.Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. It is present in all forms of carbon-based life, and in the human body carbon is the second most abundant element by mass (about 18.5%) after oxygen. This abundance, together with the unique diversity of organic compounds and their unusual polymer-forming ability at the temperatures commonly encountered on Earth, make this element the chemical basis of all known life.The atoms of carbon can be bonded together in different ways: allotropes of carbon. The best known are graphite, diamond, and amorphous carbon. The physical properties of carbon vary widely with the allotropic form. For example, graphite is opaque and black, while diamond is highly transparent. Graphite is soft enough to form a streak on paper (hence its name, from the Greek word ""γράφω"" which means ""to write""), while diamond is the hardest naturally-occurring material known. Graphite is a very good conductor, while diamond has a very low electrical conductivity. Under normal conditions, diamond, carbon nanotubes, and graphene have the highest thermal conductivities of all known materials. All carbon allotropes are solids under normal conditions, with graphite being the most thermodynamically stable form. They are chemically resistant and require high temperature to react even with oxygen.The most common oxidation state of carbon in inorganic compounds is +4, while +2 is found in carbon monoxide and other transition metal carbonyl complexes. The largest sources of inorganic carbon are limestones, dolomites and carbon dioxide, but significant quantities occur in organic deposits of coal, peat, oil and methane clathrates. Carbon forms a vast number of compounds, more than any other element, with almost ten million compounds described to date, which in turn are a tiny fraction of such compounds that are theoretically possible under standard conditions.