Chapter 6
... • The energy level number in front of (s) is always the period number • The energy level number in front of the (p) is the period number • The energy level number in front of the (d) is one less than the period number • The energy level number in front of the (f) is 2 less than the period number ...
... • The energy level number in front of (s) is always the period number • The energy level number in front of the (p) is the period number • The energy level number in front of the (d) is one less than the period number • The energy level number in front of the (f) is 2 less than the period number ...
The Periodic Table
... • Many new elements were discovered in the 1800s – electricity allowed compounds to be broken into elements – industrial revolution led to chemistry based industries – spectrometer allowed elements to be identified ...
... • Many new elements were discovered in the 1800s – electricity allowed compounds to be broken into elements – industrial revolution led to chemistry based industries – spectrometer allowed elements to be identified ...
The ocean is a mixture.
... in the B families. These are the metals you are probably most familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. ...
... in the B families. These are the metals you are probably most familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. ...
Section A oxide in molten cryolite?
... C cryolite reacts with the aluminium oxide to form ions D molten aluminium oxide alone would not conduct electricity Q2 A cheap carbon monoxide detector for a gas heater consists of a patch containing palladium chloride crystals. When carbon monoxide is present, the crystals turn from orange to blac ...
... C cryolite reacts with the aluminium oxide to form ions D molten aluminium oxide alone would not conduct electricity Q2 A cheap carbon monoxide detector for a gas heater consists of a patch containing palladium chloride crystals. When carbon monoxide is present, the crystals turn from orange to blac ...
Chemical-Periodicity
... properties after arranging by increasing atomic mass. • Eventually led to grouping by similar properties side by side ...
... properties after arranging by increasing atomic mass. • Eventually led to grouping by similar properties side by side ...
Metals and Non
... shell of highest principal quantum number. Removal of an electron(s) from a metal atom occurs without great difficulty, producing a positive ion (cation). Metals generally are malleable, and ductile solids with a lustrous appearance and an ability to conduct heat and electricity. Found to the left o ...
... shell of highest principal quantum number. Removal of an electron(s) from a metal atom occurs without great difficulty, producing a positive ion (cation). Metals generally are malleable, and ductile solids with a lustrous appearance and an ability to conduct heat and electricity. Found to the left o ...
The Periodic Table - Ms. Dormer
... Mendeleev’s table contains gaps that unknown elements should fill He predicted the properties of these unknown elements & gave them names ...
... Mendeleev’s table contains gaps that unknown elements should fill He predicted the properties of these unknown elements & gave them names ...
ExamView - chemistry chapter 6 test.tst
... d. liquids. ____ 14. Mendeleev arranged the known chemical elements in a table according to increasing a. mass. c. atomic number. b. number of electrons. d. number of protons. ____ 15. What is the element with the highest electronegativity value? a. helium c. calcium b. cesium d. fluorine ____ 16. O ...
... d. liquids. ____ 14. Mendeleev arranged the known chemical elements in a table according to increasing a. mass. c. atomic number. b. number of electrons. d. number of protons. ____ 15. What is the element with the highest electronegativity value? a. helium c. calcium b. cesium d. fluorine ____ 16. O ...
Name: Date: Period: ______ Graphing Periodic Trends Purpose:To
... 11. Bb, Cc, and Dd were all named for planets, but the planet for which Cc was named is now no longer considered to be a planet. Of these three elements, only Dd is naturallyoccurring and it is also an alpha decay product of Cc. They were all discovered at the University of California at Berkeley. I ...
... 11. Bb, Cc, and Dd were all named for planets, but the planet for which Cc was named is now no longer considered to be a planet. Of these three elements, only Dd is naturallyoccurring and it is also an alpha decay product of Cc. They were all discovered at the University of California at Berkeley. I ...
The Periodic Table - Science Education at Jefferson Lab
... • They are never found uncombined in nature. • They have two valence electrons. • Alkaline earth metals include magnesium and calcium, among others. ...
... • They are never found uncombined in nature. • They have two valence electrons. • Alkaline earth metals include magnesium and calcium, among others. ...
Groups
... Group 8A or 18: Noble Gases cont. Properties 1. all are gases at room temp 2. relatively unreactive Part of the p -block They have no common ionic charge because they don’t form ions, have stable electron ...
... Group 8A or 18: Noble Gases cont. Properties 1. all are gases at room temp 2. relatively unreactive Part of the p -block They have no common ionic charge because they don’t form ions, have stable electron ...
The Periodic Table
... (The Octet Rule). As a rule, metals lose valence electrons and non-metals gain electrons to form ions. The dependable oxidation numbers are defined by “Dr. May’s Always Rule”: Alkali Metals are +1, Alkaline Earth Metals are +2, The Boron Family elements are +3, The Oxygen Family elements are –2, and ...
... (The Octet Rule). As a rule, metals lose valence electrons and non-metals gain electrons to form ions. The dependable oxidation numbers are defined by “Dr. May’s Always Rule”: Alkali Metals are +1, Alkaline Earth Metals are +2, The Boron Family elements are +3, The Oxygen Family elements are –2, and ...
Matter: Building Blocks of the Universe Chapter 5 Classification of
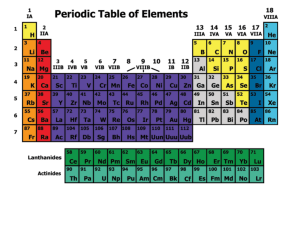
... the same number of valence electrons Family 1—Alkali—soft, silver, white, shiny—react or combine with other elements easily—never found alone in nature Family 2—Alkaline—Earth metals—very reactive Between Family 2 and 13 are the transition metals—these are the metals you are most familiar with ...
... the same number of valence electrons Family 1—Alkali—soft, silver, white, shiny—react or combine with other elements easily—never found alone in nature Family 2—Alkaline—Earth metals—very reactive Between Family 2 and 13 are the transition metals—these are the metals you are most familiar with ...
Part D Questions and Problems
... filled first and second levels. When chlorine reacts, it gains an electron in its highest occupied energy level. An ion with three occupied energy levels is larger than an ion with two occupied energy levels. 4. Across a period from left to right the principal energy level remains the same, but the ...
... filled first and second levels. When chlorine reacts, it gains an electron in its highest occupied energy level. An ion with three occupied energy levels is larger than an ion with two occupied energy levels. 4. Across a period from left to right the principal energy level remains the same, but the ...
PERIODIC TABLE - WordPress.com
... Read pages 64-69 from your textbook [Chapter 3. Elements and Compounds, Section 3.1] and answer the following questions: 1. Which property of elements did Mendeleev use to arrange elements in his periodic table? 2. State three physical properties of metals. 3. What is atomic number? 4. What are the ...
... Read pages 64-69 from your textbook [Chapter 3. Elements and Compounds, Section 3.1] and answer the following questions: 1. Which property of elements did Mendeleev use to arrange elements in his periodic table? 2. State three physical properties of metals. 3. What is atomic number? 4. What are the ...
Atomic
... IONIC Compounds (metal + nonmetal) - The symbol for the positive ion (metal) is written first - The symbol for the negative ion (non-metal) is written second. - The charges on each ion are criss-crossed and used as subscripts for the formula but the positive and negative signs are never used in the ...
... IONIC Compounds (metal + nonmetal) - The symbol for the positive ion (metal) is written first - The symbol for the negative ion (non-metal) is written second. - The charges on each ion are criss-crossed and used as subscripts for the formula but the positive and negative signs are never used in the ...
Transitional metals By Brianna Falconer Danisha Brown Dylan Neary
... 29 and it also has a very high chemical and electronically conductivity Gold- gold is a Latin word its atomic number is 79 it is precious metal and it is used for jewelry Mercury- it is also known as quick silver. it is a chemical element its atomic number is 80 and it is also a heavy silver ...
... 29 and it also has a very high chemical and electronically conductivity Gold- gold is a Latin word its atomic number is 79 it is precious metal and it is used for jewelry Mercury- it is also known as quick silver. it is a chemical element its atomic number is 80 and it is also a heavy silver ...
Exam Review - hrsbstaff.ednet.ns.ca
... The reaction of solutions of ammonium phosphate and barium nitrate gives a precipitate of barium phosphate. The equation that best represents this statement is a) 2(NH4)3PO4(s) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(s). b) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(s) + 6NH4NO3(aq). c) 2(NH4)3PO4 ...
... The reaction of solutions of ammonium phosphate and barium nitrate gives a precipitate of barium phosphate. The equation that best represents this statement is a) 2(NH4)3PO4(s) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(s). b) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(s) + 6NH4NO3(aq). c) 2(NH4)3PO4 ...
SCH3U Periodic Table Worksheet 1. Where are the most active
... nucleus pulling electrons in closer. 4. As you travel down a group, the atomic radius (increases/decreases). Why? Increases. Adding extra shells – the inner shells shield the pull from the nucleus, thus making the radius larger and larger. 5. A negative ion is (larger/smaller) than its parent atom. ...
... nucleus pulling electrons in closer. 4. As you travel down a group, the atomic radius (increases/decreases). Why? Increases. Adding extra shells – the inner shells shield the pull from the nucleus, thus making the radius larger and larger. 5. A negative ion is (larger/smaller) than its parent atom. ...
Complete the following equations
... While ionization energy generally increases from left to right across period in the periodic table, a certain anomaly is observed in this trend. For example, in the second period, ionization energy decreases from Be to B and from N to O; in the third period, ionization energy decreases from Mg to Al ...
... While ionization energy generally increases from left to right across period in the periodic table, a certain anomaly is observed in this trend. For example, in the second period, ionization energy decreases from Be to B and from N to O; in the third period, ionization energy decreases from Mg to Al ...
01.CN_Other pages/p1-9
... • Learn the method of isolating useful materials from minerals, for example, the extraction of metals from their ores. • Recognize that limestone, chalk and marble are different forms of calcium carbonate. • Study the weathering and erosion of rocks. • Explore the thermal decomposition of calcium ca ...
... • Learn the method of isolating useful materials from minerals, for example, the extraction of metals from their ores. • Recognize that limestone, chalk and marble are different forms of calcium carbonate. • Study the weathering and erosion of rocks. • Explore the thermal decomposition of calcium ca ...
















![5-2%20Using%20the%20Periodic%20Table[1]](http://s1.studyres.com/store/data/004413504_1-36f532b333702bb3fb3bd75d47d5f6a8-300x300.png)