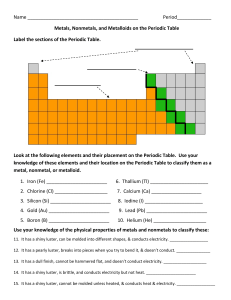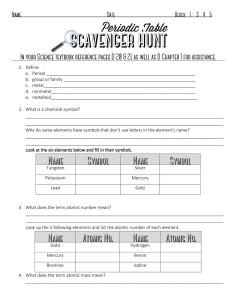
1. Define: a. Period b. group or family
... reactivity of the elements as you go left to right across the periodic table. Describe what happens to atomic mass as you go left to right across the periodic table. Describe what happens to atomic mass as you from top to bottom on a periodic table Describe what happens to atom size as you go from t ...
... reactivity of the elements as you go left to right across the periodic table. Describe what happens to atomic mass as you go left to right across the periodic table. Describe what happens to atomic mass as you from top to bottom on a periodic table Describe what happens to atom size as you go from t ...
C Carbon Cu Copper
... electrons in their very outermost energy level (This is called the rule of octet.) Atoms bond until this level is complete. Atoms with few valence electrons lose them during bonding. Atoms with 6, 7, or 8 valence electrons gain electrons during bonding. ...
... electrons in their very outermost energy level (This is called the rule of octet.) Atoms bond until this level is complete. Atoms with few valence electrons lose them during bonding. Atoms with 6, 7, or 8 valence electrons gain electrons during bonding. ...
CHAPTER 6: Earth science
... 1. The following photograph shows the result of adding a colourless solution of silver nitrate to a colourless solution of sodium chloride in a test tube. (a) A white precipitate forms. Define the term ‘precipitate’. An insoluble solid forms when two solutions are mixed. ...
... 1. The following photograph shows the result of adding a colourless solution of silver nitrate to a colourless solution of sodium chloride in a test tube. (a) A white precipitate forms. Define the term ‘precipitate’. An insoluble solid forms when two solutions are mixed. ...
U1 Periodic Trends - Alliance Ouchi-O`Donovan 6
... In this investigation, your lab group will recreate Dimitri Mendeleev’s discovery of the classification of the elements and the periodic law using a deck of special element cards. The real properties of the elements, but not their names or symbols, are written on these cards. As the cards are arra ...
... In this investigation, your lab group will recreate Dimitri Mendeleev’s discovery of the classification of the elements and the periodic law using a deck of special element cards. The real properties of the elements, but not their names or symbols, are written on these cards. As the cards are arra ...
Alkali metals
... • The halogens are typical nonmetals. • They have highly negative electron affinities, so they exist as anions in nature. • They react directly with metals to form metal ...
... • The halogens are typical nonmetals. • They have highly negative electron affinities, so they exist as anions in nature. • They react directly with metals to form metal ...
Periodic Table – an arrangement of the elements in order of their
... Period – a horizontal row of elements in the periodic table. Alkali metal – an element in Group 1a of the periodic table. Alkaline earth metal – an element in Group 2a of the periodic table. Halogen – a reactive nonmetallic element in Group 7a of the periodic table. Noble gas – an inactive element i ...
... Period – a horizontal row of elements in the periodic table. Alkali metal – an element in Group 1a of the periodic table. Alkaline earth metal – an element in Group 2a of the periodic table. Halogen – a reactive nonmetallic element in Group 7a of the periodic table. Noble gas – an inactive element i ...
AS Chemistry - Crawshaw Academy
... be comfortable with the basic Chemistry from the GCSE course, most significantly: ‘Bonding and Structure’, ‘Periodicity’, ‘Chemical Formulae’, Chemistry Calculations’ and ‘Balancing Equations’. In order for you to settle into the course quickly it is essential that you do some background work on the ...
... be comfortable with the basic Chemistry from the GCSE course, most significantly: ‘Bonding and Structure’, ‘Periodicity’, ‘Chemical Formulae’, Chemistry Calculations’ and ‘Balancing Equations’. In order for you to settle into the course quickly it is essential that you do some background work on the ...
Balancing Equations
... Remember, each element has its own symbol(s) - all found in the periodic table ...
... Remember, each element has its own symbol(s) - all found in the periodic table ...
Name Period_____________ Metals, Nonmetals, and Metalloids on
... 13. It has a dull finish, cannot be hammered flat, and doesn’t conduct electricity. ___________________ 14. It has a shiny luster, is brittle, and conducts electricity but not heat. ______________________ 15. It has a shiny luster, cannot be molded unless heated, & conducts heat & electricity. _____ ...
... 13. It has a dull finish, cannot be hammered flat, and doesn’t conduct electricity. ___________________ 14. It has a shiny luster, is brittle, and conducts electricity but not heat. ______________________ 15. It has a shiny luster, cannot be molded unless heated, & conducts heat & electricity. _____ ...
Unit 2 Exam Review: Matter and its Properties This review does not
... 25. Without using your notes, attempt to fill in the blank periodic table below. Label the alkali metals, alkaline earth metals, halogens, noble gases, and transition metals. Write in the group numbers and the number of valence electrons for the main groups. Draw in the staircase and write in where ...
... 25. Without using your notes, attempt to fill in the blank periodic table below. Label the alkali metals, alkaline earth metals, halogens, noble gases, and transition metals. Write in the group numbers and the number of valence electrons for the main groups. Draw in the staircase and write in where ...
Water Metal Hydroxide + Hydrogen
... Table is also explored in Unit 1/2. On the outline of the Periodic Table below, use three different colours to shade the elements that are ...
... Table is also explored in Unit 1/2. On the outline of the Periodic Table below, use three different colours to shade the elements that are ...
Study Guide Atoms and Periodic Table TEST Nov 21st
... 1. The scientists responsible for developing and arranging the periodic table and how they arranged it. 2. Difference between a period and group on the periodic table 3. Can you locate an element if given the period and group number? 4. Where metals, non metals and metalloids are found and identify ...
... 1. The scientists responsible for developing and arranging the periodic table and how they arranged it. 2. Difference between a period and group on the periodic table 3. Can you locate an element if given the period and group number? 4. Where metals, non metals and metalloids are found and identify ...
Topics 3 and 13 Outline
... • The terms alkali metals, halogens, noble gases, transition metals, lanthanoids and actinoids should be known. • The group numbering scheme from group 1 to group 18, as recommended by IUPAC, should be used. 3.2 Periodic trends Essential idea: Elements show trends in their physical and chemical prop ...
... • The terms alkali metals, halogens, noble gases, transition metals, lanthanoids and actinoids should be known. • The group numbering scheme from group 1 to group 18, as recommended by IUPAC, should be used. 3.2 Periodic trends Essential idea: Elements show trends in their physical and chemical prop ...
Electron Energy Level
... Easily lose valence electron (Reducing agents) React violently with water Large hydration energy React with halogens to form salts (valence electrons are electrons in the outermost shell of an atom) ...
... Easily lose valence electron (Reducing agents) React violently with water Large hydration energy React with halogens to form salts (valence electrons are electrons in the outermost shell of an atom) ...
Periodic Table Test Chemistry 1 1. What is the horizontal row in the
... 1. What is the horizontal row in the periodic table? 2. What is the vertical column in the periodic table? 3. What states that the repetition of properties occurs when elements are arranged in order of increasing atomic number? 4. What type of element is a good conductor of heat and electric current ...
... 1. What is the horizontal row in the periodic table? 2. What is the vertical column in the periodic table? 3. What states that the repetition of properties occurs when elements are arranged in order of increasing atomic number? 4. What type of element is a good conductor of heat and electric current ...
Development of the Periodic Table
... number) The vertical columns are called groups or families (organized by chemical properties) Periodic Law: when the elements are arranged in order of increasing atomic number, there is a periodic pattern in their physical and chemical properties ...
... number) The vertical columns are called groups or families (organized by chemical properties) Periodic Law: when the elements are arranged in order of increasing atomic number, there is a periodic pattern in their physical and chemical properties ...
The Periodic Table
... • An airship is a buoyant aircraft that can be steered and propelled through the air. Unlike aerodynamic craft (e.g. airplanes and helicopters) which stay aloft by moving an airfoil through the air in order to produce lift, aerostatic craft such as airships (and balloons) stay aloft primarily by me ...
... • An airship is a buoyant aircraft that can be steered and propelled through the air. Unlike aerodynamic craft (e.g. airplanes and helicopters) which stay aloft by moving an airfoil through the air in order to produce lift, aerostatic craft such as airships (and balloons) stay aloft primarily by me ...
AP Chemistry Summer Assignment 2016 revised
... Calculate the experimental percent of water in the compound. 28. How do you distinguish: Use a specific example to show the difference? a. An element from a compound. b. An element from a mixture. c. A true solution from a heterogeneous mixture. d. Distillation from filtration. e. Chromatography fr ...
... Calculate the experimental percent of water in the compound. 28. How do you distinguish: Use a specific example to show the difference? a. An element from a compound. b. An element from a mixture. c. A true solution from a heterogeneous mixture. d. Distillation from filtration. e. Chromatography fr ...
AP Chemistry Chapter 7
... Alkaline Earth Metals • They are never found uncombined in nature. • They have two valence electrons. • Alkaline earth metals include magnesium and calcium, among others. ...
... Alkaline Earth Metals • They are never found uncombined in nature. • They have two valence electrons. • Alkaline earth metals include magnesium and calcium, among others. ...
Unit One Periodicity of Elements and their Properties
... 3. Magnesium is less active than calcium , while it is more active than barium. 4. Alkali metals are bad conductors of heat. ...
... 3. Magnesium is less active than calcium , while it is more active than barium. 4. Alkali metals are bad conductors of heat. ...
Complete the following equations
... the seawater sample of part (a) in order to precipitate all of Mg2+ as magnesium hydroxide. (c) Write a balanced equation for reaction that involves Mg2+(aq), CaO(s), and H2O to produce Mg(OH)2(s). ...
... the seawater sample of part (a) in order to precipitate all of Mg2+ as magnesium hydroxide. (c) Write a balanced equation for reaction that involves Mg2+(aq), CaO(s), and H2O to produce Mg(OH)2(s). ...
In the periodic table, the elements are placed from left to right in
... A group, or family, is a vertical column in the periodic table. Elements in the same group show patterns in atomic radius,ionization energy, and electronegativity. From top to bottom in a group, the atomic radii of the elements increase: since there are more filled energy levels, valence electrons a ...
... A group, or family, is a vertical column in the periodic table. Elements in the same group show patterns in atomic radius,ionization energy, and electronegativity. From top to bottom in a group, the atomic radii of the elements increase: since there are more filled energy levels, valence electrons a ...
Re-typed from The Ultimate Chemical Equations Handbook by
... Chemists write balanced equations to illustrate what is happening during a chemical reaction. Bonds are broken, atoms are rearranged, and new bonds are formed. Every chemical reaction supports the Law of conservation of Matter. This means that in every reaction, the number of atoms of each type of e ...
... Chemists write balanced equations to illustrate what is happening during a chemical reaction. Bonds are broken, atoms are rearranged, and new bonds are formed. Every chemical reaction supports the Law of conservation of Matter. This means that in every reaction, the number of atoms of each type of e ...