Nanochemistry Lecture 1
... What’s special with Nano? The properties of nanomaterials deviate from those of single crystals or polycrystals (bulk). For example, the fundamental properties like electronic, magnetic, optical, chemical and biological Surface properties: energy levels, electronic structure, and reactivity are diff ...
... What’s special with Nano? The properties of nanomaterials deviate from those of single crystals or polycrystals (bulk). For example, the fundamental properties like electronic, magnetic, optical, chemical and biological Surface properties: energy levels, electronic structure, and reactivity are diff ...
Module 1
... Electrons leave the cathode Holes move from anode + ve holes are much more mobile than – ve electrons • So holes moves across from HTL to emissive layer • Recombination of electron hole pair leads to creation of a photon with frequency between LUMO & HOMO levels ...
... Electrons leave the cathode Holes move from anode + ve holes are much more mobile than – ve electrons • So holes moves across from HTL to emissive layer • Recombination of electron hole pair leads to creation of a photon with frequency between LUMO & HOMO levels ...
Shaping the color - University of Guelph
... (a) This photonic waveguide formed from a thin silicon membrane contains a triangular lattice of air holes separated by 300 nm. (b) A plot of transmission versus wavelength for the device shows that it has a photonic band gap between 725 nm and 825 nm if the electric field associated with the elect ...
... (a) This photonic waveguide formed from a thin silicon membrane contains a triangular lattice of air holes separated by 300 nm. (b) A plot of transmission versus wavelength for the device shows that it has a photonic band gap between 725 nm and 825 nm if the electric field associated with the elect ...
UDC 621
... On fig. 2 classification of crystals by a power range of their electrons is presented in a bit different look (see fig. 1). Zones of an electronic range allow to construct models of various options of electronic ranges of crystals for three main cases: – zones of an electric range of electrons are n ...
... On fig. 2 classification of crystals by a power range of their electrons is presented in a bit different look (see fig. 1). Zones of an electronic range allow to construct models of various options of electronic ranges of crystals for three main cases: – zones of an electric range of electrons are n ...
Student worksheet on..
... electron microscope (SEM). The SEM has helped to open up the world at the nano-level (10-9 m or nanometre (nm) level). An SEM was created/invented in the mid-to-latter part of the 20th century. The SEM has a resolution down to 10 nm (10 x 10-9 m) and has a much greater (focused) depth of field than ...
... electron microscope (SEM). The SEM has helped to open up the world at the nano-level (10-9 m or nanometre (nm) level). An SEM was created/invented in the mid-to-latter part of the 20th century. The SEM has a resolution down to 10 nm (10 x 10-9 m) and has a much greater (focused) depth of field than ...
Effects of Toxic Materials
... This means that some alloys of aluminium increase in hardness and strength over a period of a few days when they have been quenched from high temperatures. ...
... This means that some alloys of aluminium increase in hardness and strength over a period of a few days when they have been quenched from high temperatures. ...
Chapter 23
... the material Examples of good conductors include copper, aluminum and silver When a good conductor is charged in a small region, the charge readily distributes itself over the entire surface of the material ...
... the material Examples of good conductors include copper, aluminum and silver When a good conductor is charged in a small region, the charge readily distributes itself over the entire surface of the material ...
Ceramic Glass
... each other. As the melt cools, thermal vibrational energy decreases and the chains can’t move as easily so the structure becomes more rigid. Silica is the most important constituent of glass, but other oxides are added to change certain physical characteristics or to lower the melting point. Glass i ...
... each other. As the melt cools, thermal vibrational energy decreases and the chains can’t move as easily so the structure becomes more rigid. Silica is the most important constituent of glass, but other oxides are added to change certain physical characteristics or to lower the melting point. Glass i ...
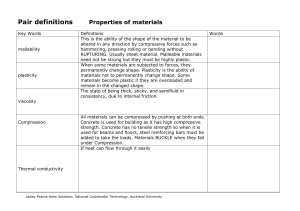
Pair definitions Properties of materials
... permanently change shape. Plasticity is the ability of materials not to permanently change shape. Some materials become plastic if they are overloaded and remain in the changed shape. The state of being thick, sticky, and semifluid in consistency, due to internal friction. ...
... permanently change shape. Plasticity is the ability of materials not to permanently change shape. Some materials become plastic if they are overloaded and remain in the changed shape. The state of being thick, sticky, and semifluid in consistency, due to internal friction. ...
Ch 1: Engineering materials
... Troom data cannot be used in design calculation if the part will be used at other temp. Long term properties cannot be predicted from short term prop. Eg. Creep behavior Engineering plastics are britle (notched impact strength < 5.4 J/cm) Avoid stress raiser ...
... Troom data cannot be used in design calculation if the part will be used at other temp. Long term properties cannot be predicted from short term prop. Eg. Creep behavior Engineering plastics are britle (notched impact strength < 5.4 J/cm) Avoid stress raiser ...
About Magnetism - Georgetown College
... But what is electricity? Electricity comes from moving electrons. In metal wires the electrons jump from atom to atom along the wire. In lightening, the electrons hop from water droplet to water droplet across the air, or to the ground. This is the origin of the term “ground” in reference to electri ...
... But what is electricity? Electricity comes from moving electrons. In metal wires the electrons jump from atom to atom along the wire. In lightening, the electrons hop from water droplet to water droplet across the air, or to the ground. This is the origin of the term “ground” in reference to electri ...
Pyroelectric Effect. Primary Pyroelectricity. Secondary Pyroelectricity
... Seebeck and Peltier Effects. Seebeck effect: Thermally induced electric currents in circuits of dissimilar material. Peltier effect: absorption of heat when an electric current cross a junction two dissimilar materials The dissimilar materials can be different species, or the the same species in dif ...
... Seebeck and Peltier Effects. Seebeck effect: Thermally induced electric currents in circuits of dissimilar material. Peltier effect: absorption of heat when an electric current cross a junction two dissimilar materials The dissimilar materials can be different species, or the the same species in dif ...
1-1 electric charge
... -DIFFERENT WIRES HAVE DIFFERENT RESISTANCES. COPPER HAS LESS RESISTANCE THEN IRON AND THEREFOR IS A BETTER CONDUCTOR. -RESISTANCE IS NOT A BAD THING. IT IS ACTUALLY NECESSARY. LIGHTBULBS GIVE OFF LIGHT AND HEAT DUE TO THE RESISTANCE OF THE METAL FILAMENT. THE FILAMENT IS A VERY THIN PIECE OF METAL T ...
... -DIFFERENT WIRES HAVE DIFFERENT RESISTANCES. COPPER HAS LESS RESISTANCE THEN IRON AND THEREFOR IS A BETTER CONDUCTOR. -RESISTANCE IS NOT A BAD THING. IT IS ACTUALLY NECESSARY. LIGHTBULBS GIVE OFF LIGHT AND HEAT DUE TO THE RESISTANCE OF THE METAL FILAMENT. THE FILAMENT IS A VERY THIN PIECE OF METAL T ...
Physical properties
... dispersion, so objects cannot be seen clearly through it, e.g. Tooth enamel, dental ceramics. Transparency: Is the property of a material that allows passage of light with only little distortion that objects can be seen clearly ...
... dispersion, so objects cannot be seen clearly through it, e.g. Tooth enamel, dental ceramics. Transparency: Is the property of a material that allows passage of light with only little distortion that objects can be seen clearly ...
Chapter 4 Chapter Review Question Answers A. Radio waves (long
... 24. 2s orbital is higher in energy for the electrons than the 1s orbital. 2s orbital is further from the nucleus. 2s orbital is larger. 25. They would be at right angles to each other. 26. A. Electrons occupy the lowest energy orbital they can. b. Multi-electron atoms will have electrons filling th ...
... 24. 2s orbital is higher in energy for the electrons than the 1s orbital. 2s orbital is further from the nucleus. 2s orbital is larger. 25. They would be at right angles to each other. 26. A. Electrons occupy the lowest energy orbital they can. b. Multi-electron atoms will have electrons filling th ...
Lecture 29
... μ0 = 4π × 10-7 N/A2, the permeability of free space. This linear relation between M and B is always pretty well satisfied in paramagnets. Ferromagnets Ferromagnets have a moment M even in the absence of a field. They are so-called because iron is one of them. For the first thousand years, the study ...
... μ0 = 4π × 10-7 N/A2, the permeability of free space. This linear relation between M and B is always pretty well satisfied in paramagnets. Ferromagnets Ferromagnets have a moment M even in the absence of a field. They are so-called because iron is one of them. For the first thousand years, the study ...
Teacher`s Notes
... conductor - a material through which electricity can readily flow insulator - a material through which electricity cannot readily flow Lesson Notes: 1. Worksheet item 1 asks students to think of practical application of electricity that they have come in contact with. 2. Understanding the main parts ...
... conductor - a material through which electricity can readily flow insulator - a material through which electricity cannot readily flow Lesson Notes: 1. Worksheet item 1 asks students to think of practical application of electricity that they have come in contact with. 2. Understanding the main parts ...
1.
... 3. Semiconductor Materials and Properties Semiconductor materials, Elemental and compound semiconductors, Band model of semiconductors, Carrier concentration in energy bands, Fermi level and energy distribution of carriers inside band, extrinsic semiconductors, concept of effective mass, heavily dop ...
... 3. Semiconductor Materials and Properties Semiconductor materials, Elemental and compound semiconductors, Band model of semiconductors, Carrier concentration in energy bands, Fermi level and energy distribution of carriers inside band, extrinsic semiconductors, concept of effective mass, heavily dop ...
Plum pudding
... Problem 1a.- The plum pudding analogy hides the fact that Thomson thought the electrons were moving inside the atom. Nevertheless let’s consider a plum pudding model of a lithium ion (Li+): A spherical volume of radius R with homogeneously distributed positive charge = +3e and two electrons, with ch ...
... Problem 1a.- The plum pudding analogy hides the fact that Thomson thought the electrons were moving inside the atom. Nevertheless let’s consider a plum pudding model of a lithium ion (Li+): A spherical volume of radius R with homogeneously distributed positive charge = +3e and two electrons, with ch ...
12021 14115 3 Hours / 100 Marks Seat No.
... c) Distinguish between P-type and N-type semiconductor. d) Give one application each of nanotechnology in electronics, automobile. e) Define forbidden energy gap. Draw energy band diagram for conductor semiconductor and insulators. f) ...
... c) Distinguish between P-type and N-type semiconductor. d) Give one application each of nanotechnology in electronics, automobile. e) Define forbidden energy gap. Draw energy band diagram for conductor semiconductor and insulators. f) ...
PHYSICS 100 ELECTROSTATICS
... positive and negative charge. It was observed that rubbing two materials together sometimes resulted in both materials acquiring opposite net charges. In this process, the outermost or valence electrons in one material were transferred to the second material. The first material would be charged posi ...
... positive and negative charge. It was observed that rubbing two materials together sometimes resulted in both materials acquiring opposite net charges. In this process, the outermost or valence electrons in one material were transferred to the second material. The first material would be charged posi ...
Untitled
... Another example for Covalent network solids • Silicon – Covalent network solid and semiconductor – Forms a 3-D network similar to carbon – Doping (adding slight impurities by using a different element in the network solid) silicon’s covalent network structure can increase it conductivity ...
... Another example for Covalent network solids • Silicon – Covalent network solid and semiconductor – Forms a 3-D network similar to carbon – Doping (adding slight impurities by using a different element in the network solid) silicon’s covalent network structure can increase it conductivity ...
Materials and their Properties
... • Ceramics are very heat resistant and this makea them appropriate for kitchen appliances such as ovens and heating dishes. • Ceramics do not corrode and therefore are very useful for ducts and drains. • Just the same, ceramics are fragile and can be easily broken. Care must be taken in the choice ...
... • Ceramics are very heat resistant and this makea them appropriate for kitchen appliances such as ovens and heating dishes. • Ceramics do not corrode and therefore are very useful for ducts and drains. • Just the same, ceramics are fragile and can be easily broken. Care must be taken in the choice ...
Improved ferroelectric materials - structural
... stabilized by the Ba2+ cations (Fig. 1). Various doping studies have been carried out in BaTiO3 with an objective to improve its properties. For example, rare earth elements such as cerium (Ce) and lanthanum (La) have been partially doped in the Barium site to shift the dielectric maximum towards ro ...
... stabilized by the Ba2+ cations (Fig. 1). Various doping studies have been carried out in BaTiO3 with an objective to improve its properties. For example, rare earth elements such as cerium (Ce) and lanthanum (La) have been partially doped in the Barium site to shift the dielectric maximum towards ro ...
Semiconductor
A semiconductor material has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Semiconductors are the foundation of modern electronics. Semiconducting materials exist in two types - elemental materials and compound materials. The modern understanding of the properties of a semiconductor relies on quantum physics to explain the movement of electrons and holes in a crystal lattice. The unique arrangement of the crystal lattice makes silicon and germanium the most commonly used elements in the preparation of semiconducting materials. An increased knowledge of semiconductor materials and fabrication processes has made possible continuing increases in the complexity and speed of microprocessors and memory devices. Some of the information on this page may be outdated within a year because new discoveries are made in the field frequently.The electrical conductivity of a semiconductor material increases with increasing temperature, which is behaviour opposite to that of a metal. Semiconductor devices can display a range of useful properties such as passing current more easily in one direction than the other, showing variable resistance, and sensitivity to light or heat. Because the electrical properties of a semiconductor material can be modified by controlled addition of impurities, or by the application of electrical fields or light, devices made from semiconductors can be used for amplification, switching, and energy conversion.Current conduction in a semiconductor occurs through the movement of free electrons and ""holes"", collectively known as charge carriers. Adding impurity atoms to a semiconducting material, known as ""doping"", greatly increases the number of charge carriers within it. When a doped semiconductor contains mostly free holes it is called ""p-type"", and when it contains mostly free electrons it is known as ""n-type"". The semiconductor materials used in electronic devices are doped under precise conditions to control the concentration and regions of p- and n-type dopants. A single semiconductor crystal can have many p- and n-type regions; the p–n junctions between these regions are responsible for the useful electronic behaviour.Some of the properties of semiconductor materials were observed throughout the mid 19th and first decades of the 20th century. Development of quantum physics in turn allowed the development of the transistor in 1947. Although some pure elements and many compounds display semiconductor properties, silicon, germanium, and compounds of gallium are the most widely used in electronic devices. Elements near the so-called ""metalloid staircase"", where the metalloids are located on the periodic table, are usually used as semiconductors.The nickname of the southern area of Northern California is Silicon Valley because of all the influential tech companies that have their headquarters there. An integral part of today’s technology is built upon semiconductors, which are made primarily of silicon. Some major companies include Marvell Technology Group, National Semiconductor (now part of Texas Instruments), and Advanced Micro Devices.