Chapter 20 - NUS Physics Department
... right. The hot water can freeze faster than the colder water in some cases due to two effects. First, the hot water does actually lose heat faster initially since the temperature difference between the hot water and air is greater than that between the colder water and the air. However the second ef ...
... right. The hot water can freeze faster than the colder water in some cases due to two effects. First, the hot water does actually lose heat faster initially since the temperature difference between the hot water and air is greater than that between the colder water and the air. However the second ef ...
Heat Effects - Association of Chemical Engineering Students
... Heat effects discussed so far have been for physical processes. Chemical reactions also are accompanied either by the transfer of heat or by temperature changes during the course of reaction-in some cases by both. These effects are manifestations of the differences in molecular structure, and theref ...
... Heat effects discussed so far have been for physical processes. Chemical reactions also are accompanied either by the transfer of heat or by temperature changes during the course of reaction-in some cases by both. These effects are manifestations of the differences in molecular structure, and theref ...
The First Law of Thermodynamics
... For certain indices n, the process will be synonymous with other processes: if n = 0, then PV0=P=const and it is an isobaric process (constant pressure) if n = 1, then for an ideal gas PV= const and it is an isothermal process (constant ...
... For certain indices n, the process will be synonymous with other processes: if n = 0, then PV0=P=const and it is an isobaric process (constant pressure) if n = 1, then for an ideal gas PV= const and it is an isothermal process (constant ...
Slide 1
... Having established how we can measure temperatures, we now need to define a reproducible temperature scale in order that thermometers can be calibrated. The triple point of water: this is used to define a standard fixed point, since liquid water, solid ice, and water vapor can coexist in thermal equ ...
... Having established how we can measure temperatures, we now need to define a reproducible temperature scale in order that thermometers can be calibrated. The triple point of water: this is used to define a standard fixed point, since liquid water, solid ice, and water vapor can coexist in thermal equ ...
The basic concepts For the purposes of physical chemistry, the
... state of the system and is independent of how that state has been prepared. In other words, it is a function of the properties that determine the current state of the system. Changing anyone of the state variables, such as the pressure, results in a change in internal energy. The internal energy is ...
... state of the system and is independent of how that state has been prepared. In other words, it is a function of the properties that determine the current state of the system. Changing anyone of the state variables, such as the pressure, results in a change in internal energy. The internal energy is ...
State Equations The Thermodynamics of State An Isentropic
... • the theoretical maximum amount of work that can be obtained from a system at a given state P1 and T1 when interacting with a reference atmosphere at the constant pressure and temperature P0 and T0 . • describes the work potential of a given system. • also referred to as “exergy”. The following obs ...
... • the theoretical maximum amount of work that can be obtained from a system at a given state P1 and T1 when interacting with a reference atmosphere at the constant pressure and temperature P0 and T0 . • describes the work potential of a given system. • also referred to as “exergy”. The following obs ...
Spring 2016 - F-Chart Software
... EES eliminates the mathematical complexity involved in solving sets of simultaneous algebraic and differential equations that are the mathematical basis of complex problems. It provides a large bank of high-accuracy thermodynamic and transport property data and a library of heat transfer functions. ...
... EES eliminates the mathematical complexity involved in solving sets of simultaneous algebraic and differential equations that are the mathematical basis of complex problems. It provides a large bank of high-accuracy thermodynamic and transport property data and a library of heat transfer functions. ...
UNIT 5 - H-W Science Website
... b. Stir the water while heating so that the probe is not resting directly on the bottom of the can. c. Continue to stir a little after extinguishing the flame to be sure the water temperature is the same throughout. 2. Using the calorimeter set-up The calorimeters have been made so that the inner ca ...
... b. Stir the water while heating so that the probe is not resting directly on the bottom of the can. c. Continue to stir a little after extinguishing the flame to be sure the water temperature is the same throughout. 2. Using the calorimeter set-up The calorimeters have been made so that the inner ca ...
Countercurrent exchange

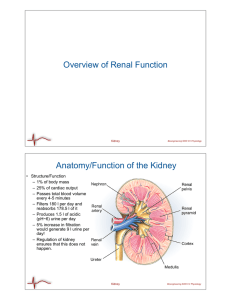
Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some component, between two flowing bodies flowing in opposite directions to each other. The flowing bodies can be liquids, gases, or even solid powders, or any combination of those. For example, in a distillation column, the vapors bubble up through the downward flowing liquid while exchanging both heat and mass.The maximum amount of heat or mass transfer that can be obtained is higher with countercurrent than co-current (parallel) exchange because countercurrent maintains a slowly declining difference or gradient (usually temperature or concentration difference). In cocurrent exchange the initial gradient is higher but falls off quickly, leading to wasted potential. For example, in the diagram at the right, the fluid being heated (exiting top) has a higher exiting temperature than the cooled fluid (exiting bottom) that was used for heating. With cocurrent or parallel exchange the heated and cooled fluids can only approach one another. The result is that countercurrent exchange can achieve a greater amount of heat or mass transfer than parallel under otherwise similar conditions. See: flow arrangement.Countercurrent exchange when set up in a circuit or loop can be used for building up concentrations, heat, or other properties of flowing liquids. Specifically when set up in a loop with a buffering liquid between the incoming and outgoing fluid running in a circuit, and with active transport pumps on the outgoing fluid's tubes, the system is called a Countercurrent multiplier, enabling a multiplied effect of many small pumps to gradually build up a large concentration in the buffer liquid.Other countercurrent exchange circuits where the incoming and outgoing fluids touch each other are used for retaining a high concentration of a dissolved substance or for retaining heat, or for allowing the external buildup of the heat or concentration at one point in the system.Countercurrent exchange circuits or loops are found extensively in nature, specifically in biologic systems. In vertebrates, they are called a Rete mirabile, originally the name of an organ in fish gills for absorbing oxygen from the water. It is mimicked in industrial systems. Countercurrent exchange is a key concept in chemical engineering thermodynamics and manufacturing processes, for example in extracting sucrose from sugar beet roots.Countercurrent multiplication is a similar but different concept where liquid moves in a loop followed by a long length of movement in opposite directions with an intermediate zone. The tube leading to the loop passively building up a gradient of heat (or cooling) or solvent concentration while the returning tube has a constant small pumping action all along it, so that a gradual intensification of the heat or concentration is created towards the loop. Countercurrent multiplication has been found in the kidneys as well as in many other biological organs.