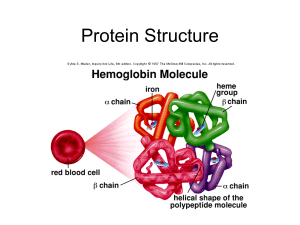
Slide 2
... Click – Protein – the big organic macromolecules made of amino acids. Proteins participate in every process within cells, they have catalytic, structural, mechanical and many other functions. The word protein comes from Greek word “proteios” which means primary! ...
... Click – Protein – the big organic macromolecules made of amino acids. Proteins participate in every process within cells, they have catalytic, structural, mechanical and many other functions. The word protein comes from Greek word “proteios” which means primary! ...
PowerPoint
... If ORF exists, then align at protein level. Amino acid substitution matrices reflect the log-odds ratio between the evolutionary and random model and can therefore help in determining homology via the alignment score. The evolutionary and random models depend on the generalized data used to derive t ...
... If ORF exists, then align at protein level. Amino acid substitution matrices reflect the log-odds ratio between the evolutionary and random model and can therefore help in determining homology via the alignment score. The evolutionary and random models depend on the generalized data used to derive t ...
History and Philosophy of Science
... • identifying anatomical structure based on CT scans; and • determining geological structure from seismic data. Pathway tools differs in that it uses classifiers to identify secondary structure in DNA sequences. SedSim, which infers models of geological structures, uses physics-based, dynamic models ...
... • identifying anatomical structure based on CT scans; and • determining geological structure from seismic data. Pathway tools differs in that it uses classifiers to identify secondary structure in DNA sequences. SedSim, which infers models of geological structures, uses physics-based, dynamic models ...
Problem 2
... Finally, a -loop was indicated, and I had no idea what one of those was either. So here it is: ...
... Finally, a -loop was indicated, and I had no idea what one of those was either. So here it is: ...
PS401 – Lec 10
... taxonomic criteria, database origin, relation to a complete genome, relation to a 3D protein structure or conserved domain. ...
... taxonomic criteria, database origin, relation to a complete genome, relation to a 3D protein structure or conserved domain. ...
Lecture 13_summary
... which can be related to amyloidosis (5-10 different sequence/predicted structural features) For example Fact : Amyloids tend to aggregare via beta sheet – Calculate: the percent of secondary structure (H,E, C) Fact : Amyloids tend to aggregate via aromatic residues Calculate : the percent of differe ...
... which can be related to amyloidosis (5-10 different sequence/predicted structural features) For example Fact : Amyloids tend to aggregare via beta sheet – Calculate: the percent of secondary structure (H,E, C) Fact : Amyloids tend to aggregate via aromatic residues Calculate : the percent of differe ...
Proteiinien merkitys - Helsingin yliopisto
... Use other data Known structure in family? Yes Comparative modelling Validate motifs against 3D model No Secondary structure prediction No: use single sequence methods No: single sequence methods Motif search Secondary structure prediction Use other data ...
... Use other data Known structure in family? Yes Comparative modelling Validate motifs against 3D model No Secondary structure prediction No: use single sequence methods No: single sequence methods Motif search Secondary structure prediction Use other data ...
Survey of Protein Structure Prediction Methods
... Trying to predict the end result of folding, using a large amount of comparison between known and unknown structures Protein folding problem Trying to understand the folding path which leads to the end result of folding, typically by MD simulations or energy ...
... Trying to predict the end result of folding, using a large amount of comparison between known and unknown structures Protein folding problem Trying to understand the folding path which leads to the end result of folding, typically by MD simulations or energy ...
E U F T DG Unfolded state, ensemble Native fold, one
... Identify regular secondary structure elements. Identify the structural units of the protein backbone. Explain why some backbone conformations are favoured and some are “forbidden” (not found in natural proteins). – Name properties on which the amino acids can be grouped. – Explain the driving forces ...
... Identify regular secondary structure elements. Identify the structural units of the protein backbone. Explain why some backbone conformations are favoured and some are “forbidden” (not found in natural proteins). – Name properties on which the amino acids can be grouped. – Explain the driving forces ...
Protein_structure_II
... Homology Modeling (Cont’d) • Accuracy of structure prediction depends on the percent amino acid sequence identity shared between the query and template. • For >50% sequence identity, RMSD (Root Mean Square Deviation) is only 1 Å for mainchain atoms, which is comparable to the accuracy of a medium-r ...
... Homology Modeling (Cont’d) • Accuracy of structure prediction depends on the percent amino acid sequence identity shared between the query and template. • For >50% sequence identity, RMSD (Root Mean Square Deviation) is only 1 Å for mainchain atoms, which is comparable to the accuracy of a medium-r ...
Cross-species Extrapolation of an Adverse Outcome Pathway for Ecdysteroid Receptor Activation
... Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS) ...
... Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS) ...
Slide 1
... Stable when incorporated into a -sheet H-bonds between peptide groups of adjacent strands Adjacent strands can be parallel or antiparallel ...
... Stable when incorporated into a -sheet H-bonds between peptide groups of adjacent strands Adjacent strands can be parallel or antiparallel ...
structures
... • Over long periods of time a sequence will acquire random mutations. – These mutations may result in a new amino acid at a given position, the deletion of an amino acid, or the introduction of a new one. – Over VERY long periods of time two sequences may diverge so much that their relationship can ...
... • Over long periods of time a sequence will acquire random mutations. – These mutations may result in a new amino acid at a given position, the deletion of an amino acid, or the introduction of a new one. – Over VERY long periods of time two sequences may diverge so much that their relationship can ...
Lecture 1: Fundamentals of Protein Structure
... Not conserved (can be many different residues in different species) ...
... Not conserved (can be many different residues in different species) ...
Proteins
... – The "R" groups get in each other's way and force a twisting of the chain into a coil. ...
... – The "R" groups get in each other's way and force a twisting of the chain into a coil. ...
Document
... experiments, just bioinformatics techniques): – Find a set of similar protein sequences to the unknown sequence ...
... experiments, just bioinformatics techniques): – Find a set of similar protein sequences to the unknown sequence ...
Text S1.
... matrices in total). PSI-BLAST was run using an expected threshold of 10 and a P-value of 0.5. PSI-BLAST was run until convergence is obtained or stopped at a maximum of 10 iterations. In each round, new hits were obtained using E-value, Max identity, and available GO annotation parameters from Table ...
... matrices in total). PSI-BLAST was run using an expected threshold of 10 and a P-value of 0.5. PSI-BLAST was run until convergence is obtained or stopped at a maximum of 10 iterations. In each round, new hits were obtained using E-value, Max identity, and available GO annotation parameters from Table ...
Beta-Sheet Structure Prediction Methods
... buried core are critical determinants of the fold. These analyses indicated strong statistical preferences for certain amino acids residues in the folded beta-structural motifs. BETAWRAP predicts beta-helix by dynamically assessing an amino acid segment into stacking beta-strands separated by variab ...
... buried core are critical determinants of the fold. These analyses indicated strong statistical preferences for certain amino acids residues in the folded beta-structural motifs. BETAWRAP predicts beta-helix by dynamically assessing an amino acid segment into stacking beta-strands separated by variab ...
TIM barrel proteins (ie
... belt of the complete proteinS4 (Fig. S2a). More generally, -barrels consist of large structures (at least 200 amino acid residues in length), predominantly composed of alternating -helices and -strands, with parallel -strands forming a “hub” surrounded by a “tire” of -helicesS5. The class ...
... belt of the complete proteinS4 (Fig. S2a). More generally, -barrels consist of large structures (at least 200 amino acid residues in length), predominantly composed of alternating -helices and -strands, with parallel -strands forming a “hub” surrounded by a “tire” of -helicesS5. The class ...
Align sequence to structure - Computational Bioscience Program
... • Threading: Align sequence to structure (templates) For each alignment, the probability that that each amino acid residue would occur in such an environment is calculated based on observed preferences in determined structures. § Rationale: • Limited number of basic folds found in nature • Amino aci ...
... • Threading: Align sequence to structure (templates) For each alignment, the probability that that each amino acid residue would occur in such an environment is calculated based on observed preferences in determined structures. § Rationale: • Limited number of basic folds found in nature • Amino aci ...
Amino acid sequence fingerprints in divergent evolution of
... e-mail: [email protected] Proteins may basically be related by divergent or convergent evolution. In divergent evolution, i.e. the evolution from a common ancestor, there is only a small percentage of residues in the amino acid sequence of a protein, that have to be conserved in order the prot ...
... e-mail: [email protected] Proteins may basically be related by divergent or convergent evolution. In divergent evolution, i.e. the evolution from a common ancestor, there is only a small percentage of residues in the amino acid sequence of a protein, that have to be conserved in order the prot ...
Proteins - Wesleyan College Faculty
... http://learn.genetics.utah.edu/content/begin/dna/transcribe/ ...
... http://learn.genetics.utah.edu/content/begin/dna/transcribe/ ...
Structural alignment

Structural alignment attempts to establish homology between two or more polymer structures based on their shape and three-dimensional conformation. This process is usually applied to protein tertiary structures but can also be used for large RNA molecules. In contrast to simple structural superposition, where at least some equivalent residues of the two structures are known, structural alignment requires no a priori knowledge of equivalent positions. Structural alignment is a valuable tool for the comparison of proteins with low sequence similarity, where evolutionary relationships between proteins cannot be easily detected by standard sequence alignment techniques. Structural alignment can therefore be used to imply evolutionary relationships between proteins that share very little common sequence. However, caution should be used in using the results as evidence for shared evolutionary ancestry because of the possible confounding effects of convergent evolution by which multiple unrelated amino acid sequences converge on a common tertiary structure.Structural alignments can compare two sequences or multiple sequences. Because these alignments rely on information about all the query sequences' three-dimensional conformations, the method can only be used on sequences where these structures are known. These are usually found by X-ray crystallography or NMR spectroscopy. It is possible to perform a structural alignment on structures produced by structure prediction methods. Indeed, evaluating such predictions often requires a structural alignment between the model and the true known structure to assess the model's quality. Structural alignments are especially useful in analyzing data from structural genomics and proteomics efforts, and they can be used as comparison points to evaluate alignments produced by purely sequence-based bioinformatics methods.The outputs of a structural alignment are a superposition of the atomic coordinate sets and a minimal root mean square deviation (RMSD) between the structures. The RMSD of two aligned structures indicates their divergence from one another. Structural alignment can be complicated by the existence of multiple protein domains within one or more of the input structures, because changes in relative orientation of the domains between two structures to be aligned can artificially inflate the RMSD.