n =1.75 n =1.4 - La Salle University
... moving with respect to this vector. Describe why you have drawn the picture this way, incorporating concepts of electrostatic force, potential energy, and potential. b) Calculate the magnitude of E. ...
... moving with respect to this vector. Describe why you have drawn the picture this way, incorporating concepts of electrostatic force, potential energy, and potential. b) Calculate the magnitude of E. ...
Biological Molecules Elements in Biological Molecules Importance
... All biological molecules are basically carbon skeletons w/ various functional groups attached –H (hydrogen: nonpolar “default” group) ...
... All biological molecules are basically carbon skeletons w/ various functional groups attached –H (hydrogen: nonpolar “default” group) ...
Organic compounds
... Draw Figure 3.5 phospholipid. Label the head and the tail. 1. How would the polar head of a phospholipid respond to water molecules? 2. How would the nonpolar tails respond to water molecules? ...
... Draw Figure 3.5 phospholipid. Label the head and the tail. 1. How would the polar head of a phospholipid respond to water molecules? 2. How would the nonpolar tails respond to water molecules? ...
First Poly(2-oxazoline)s with Pendant Amino Groups
... precursor. In most cases, the latter is advisable, since many reactive chemical groups are not compatible with the living cationic ring-opening polymerization. The poly(2-oxazoline) system is especially intriguing for the preparation of multi-functional polymers, since most 2-oxazoline monomers can ...
... precursor. In most cases, the latter is advisable, since many reactive chemical groups are not compatible with the living cationic ring-opening polymerization. The poly(2-oxazoline) system is especially intriguing for the preparation of multi-functional polymers, since most 2-oxazoline monomers can ...
1411 Practice Exam 1
... Carry out the following operations and give your answers with the correct number of significant figures (2 pts each): a) 14 + 6.724 + 0.0099 ...
... Carry out the following operations and give your answers with the correct number of significant figures (2 pts each): a) 14 + 6.724 + 0.0099 ...
Chemistry 1411 Practice Exam 1, Chapters 1
... Carry out the following operations and give your answers with the correct number of significant figures (2 pts each): a) 14 + 6.724 + 0.0099 ...
... Carry out the following operations and give your answers with the correct number of significant figures (2 pts each): a) 14 + 6.724 + 0.0099 ...
Nucleic Acids
... Monomer Units (Provide number of each required) Glycerol formula, What functional group is present? Describe the structure of the fatty acid. (Include the names of the two functional groups on each end.) ...
... Monomer Units (Provide number of each required) Glycerol formula, What functional group is present? Describe the structure of the fatty acid. (Include the names of the two functional groups on each end.) ...
synthesis of high molecular weight liquid molecular brushes and
... Matieland 7602, South Africa, #[email protected] ...
... Matieland 7602, South Africa, #[email protected] ...
ABSTRACT SYNTHESIS AND STUDY OF ELECTRO
... tetraphenylethylenes (TPEs), tetraphenylethylene based dendrimers and a series of phenyl ethers were prepared and the effect of the diarylether linkage on their electronic and optical properties was studied. Although the diarylether linkage in TPEs did not affect the properties significantly, these ...
... tetraphenylethylenes (TPEs), tetraphenylethylene based dendrimers and a series of phenyl ethers were prepared and the effect of the diarylether linkage on their electronic and optical properties was studied. Although the diarylether linkage in TPEs did not affect the properties significantly, these ...
Linear Polymer
... A pendant group on a polymer is a small group of atoms (even a small chain sometimes) that hangs off of the main chain (that is, the backbone of the polymer). ...
... A pendant group on a polymer is a small group of atoms (even a small chain sometimes) that hangs off of the main chain (that is, the backbone of the polymer). ...
Fall 2009 - ece.utah.edu
... b) The battery is hooked to a load resistor and the terminal voltage drops to 10 V. Find the value of the load resistor. ...
... b) The battery is hooked to a load resistor and the terminal voltage drops to 10 V. Find the value of the load resistor. ...
pdf - Mattson Creighton
... for how you worked each problem as well as for the correct answer. If you need more space, you may use the back of the periodic table provided — Write: “See PT” in the answer box and then hand the periodic table in with your exam. On your desk you are allowed only pencils (but no pencil pouch), an e ...
... for how you worked each problem as well as for the correct answer. If you need more space, you may use the back of the periodic table provided — Write: “See PT” in the answer box and then hand the periodic table in with your exam. On your desk you are allowed only pencils (but no pencil pouch), an e ...
Electrochemical Engineering
... HMGCC is an organisation focused on ensuring UK Government communications are highly effective, completely reliable and totally secure. As such, the Power Sources Centre (PSC) within HMGCC has a remit to research, develop and manufacture battery technology for a wide range of applications. The major ...
... HMGCC is an organisation focused on ensuring UK Government communications are highly effective, completely reliable and totally secure. As such, the Power Sources Centre (PSC) within HMGCC has a remit to research, develop and manufacture battery technology for a wide range of applications. The major ...
Chapter 5 :Structure and Function of Large Biological Molecules
... Chapter 5 :Structure and Function of Large Biological Molecules ...
... Chapter 5 :Structure and Function of Large Biological Molecules ...
1 1. (8 pts) Circle the formula (only one) that best fits each of the
... (10 pts) You have a 10.40 gram mixture of table sugar (C12H22O11) and table salt (NaCl). When this mixture is dissolved in 150 grams of water, the freezing point is –2.24oC. Calculate the percent by mass of sugar in the dry mixture. Kf = 1.86 oC kg / mol ...
... (10 pts) You have a 10.40 gram mixture of table sugar (C12H22O11) and table salt (NaCl). When this mixture is dissolved in 150 grams of water, the freezing point is –2.24oC. Calculate the percent by mass of sugar in the dry mixture. Kf = 1.86 oC kg / mol ...
twelve important naval substances – bonding
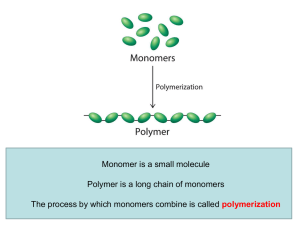
... Polymers and Biopolymers Introduction to Polymers A plastic soda bottle, a rubber band, nylon fishing line, a diaper, super glue, proteins, DNA, RNA, and complex carbohydrates are all examples of polymers. Polymer: A molecule of high molar mass formed by the joining of a large number of molecules of ...
... Polymers and Biopolymers Introduction to Polymers A plastic soda bottle, a rubber band, nylon fishing line, a diaper, super glue, proteins, DNA, RNA, and complex carbohydrates are all examples of polymers. Polymer: A molecule of high molar mass formed by the joining of a large number of molecules of ...
Honors Biology - LangdonBiology.org
... 9. The four polymers of glucose fall into two categories. Describe the two categories of carbohydrate monomers, and the two polymers in each of the categories. Glucose is the most commonly seen monosaccharide in biology. It is a hexose, a six carbon hexagonal sugar. There are two structural polymers ...
... 9. The four polymers of glucose fall into two categories. Describe the two categories of carbohydrate monomers, and the two polymers in each of the categories. Glucose is the most commonly seen monosaccharide in biology. It is a hexose, a six carbon hexagonal sugar. There are two structural polymers ...
Today*s topic is*11 letters long
... : lipid molecule formed by bonding three fatty acids to a glycerol molecule ...
... : lipid molecule formed by bonding three fatty acids to a glycerol molecule ...
Slide 1
... Urea has two amine (-NH2) groups joined by a carbonyl (C=O) functional group. Urea serves an important role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. The body uses it in many processes, most notably nitrogen e ...
... Urea has two amine (-NH2) groups joined by a carbonyl (C=O) functional group. Urea serves an important role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. The body uses it in many processes, most notably nitrogen e ...
Organic Chemistry
... Nomenclature for Alkenes 1. Root hydrocarbon name ends in -ene C2H4 is ethene 2. With more than 3 carbons, double bond is indicated by the lowest numbered carbon atom in the bond. C=CCC is 1-butene ...
... Nomenclature for Alkenes 1. Root hydrocarbon name ends in -ene C2H4 is ethene 2. With more than 3 carbons, double bond is indicated by the lowest numbered carbon atom in the bond. C=CCC is 1-butene ...
BIOCHEMISTRY - losalusd.k12.ca.us
... A carbon atom has 4 electrons in the outer most level It can form four covalent bonds with other atoms ...
... A carbon atom has 4 electrons in the outer most level It can form four covalent bonds with other atoms ...
Polythiophene

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. They can become conducting when electrons are added or removed from the conjugated π-orbitals via doping. The study of polythiophenes has intensified over the last three decades. The maturation of the field of conducting polymers was confirmed by the awarding of the 2000 Nobel Prize in Chemistry to Alan J. Heeger, Alan MacDiarmid, and Hideki Shirakawa ""for the discovery and development of conductive polymers"". The most notable property of these materials, electrical conductivity, results from the delocalization of electrons along the polymer backbone – hence the term ""synthetic metals"". However, conductivity is not the only interesting property resulting from electron delocalization. The optical properties of these materials respond to environmental stimuli, with dramatic color shifts in response to changes in solvent, temperature, applied potential, and binding to other molecules. Both color changes and conductivity changes are induced by the same mechanism—twisting of the polymer backbone, disrupting conjugation—making conjugated polymers attractive as sensors that can provide a range of optical and electronic responses.A number of comprehensive reviews have been published on PTs, the earliest dating from 1981. Schopf and Koßmehl published a comprehensive review of the literature published between 1990 and 1994. Roncali surveyed electrochemical synthesis in 1992, and the electronic properties of substituted PTs in 1997. McCullough's 1998 review focussed on chemical synthesis of conducting PTs. A general review of conjugated polymers from the 1990s was conducted by Reddinger and Reynolds in 1999. Finally, Swager et al. examined conjugated-polymer-based chemical sensors in 2000. These reviews are an excellent guide to the highlights of the primary PT literature from the last two decades.