
Organic Chemistry
... 1. For alkanes beyond butane, add -ane to the Greek root for the number of carbons. C-C-C-C-C-C = hexane 2. Alkyl substituents: drop the -ane and add -yl. -C2H5 is ethyl ...
... 1. For alkanes beyond butane, add -ane to the Greek root for the number of carbons. C-C-C-C-C-C = hexane 2. Alkyl substituents: drop the -ane and add -yl. -C2H5 is ethyl ...
FINAL EXAM
... below. For each, what is the magnetic force F on the proton? Give your answers in vector form using the unit vectors î , ...
... below. For each, what is the magnetic force F on the proton? Give your answers in vector form using the unit vectors î , ...
Structure and Properties of Polymers
... is extremely complex and is one of the most important topics in biochemistry. The chemistry of formation of macromolecules can more easily be described with synthetic polymers. Low-molecular-weight substances used for synthesis of polymers are called monomers. Because of their specific structure and ...
... is extremely complex and is one of the most important topics in biochemistry. The chemistry of formation of macromolecules can more easily be described with synthetic polymers. Low-molecular-weight substances used for synthesis of polymers are called monomers. Because of their specific structure and ...
File - Ms Hotchin SCSC
... join by amide links these are similar to polyesters in that they can form homopolymers and copolymers. • What is meant by homopolymers and copolymers? And what is the difference between functional groups in these polymers? ...
... join by amide links these are similar to polyesters in that they can form homopolymers and copolymers. • What is meant by homopolymers and copolymers? And what is the difference between functional groups in these polymers? ...
Name Strand II: Chemical Structures and Properties Multiple Choice
... b. A is a period and B is a group. d. A is a group and B is a period. ____ 10. One of the gases released after gasoline burns in a car’s engine is carbon monoxide. Which of the following statements explains why there is carbon monoxide in this mixture of gases? a. Not enough oxygen is present when g ...
... b. A is a period and B is a group. d. A is a group and B is a period. ____ 10. One of the gases released after gasoline burns in a car’s engine is carbon monoxide. Which of the following statements explains why there is carbon monoxide in this mixture of gases? a. Not enough oxygen is present when g ...
Organic chemistry
... When a carbon chain has a double (or triple bond) it does not need to have the maximum number of hydrogen's… so it is not full….. Or not saturated. When a carbon chain has only single bonds it needs the max number of hydrogen's…. It is saturated. ...
... When a carbon chain has a double (or triple bond) it does not need to have the maximum number of hydrogen's… so it is not full….. Or not saturated. When a carbon chain has only single bonds it needs the max number of hydrogen's…. It is saturated. ...
Mon Feb 15 lecture
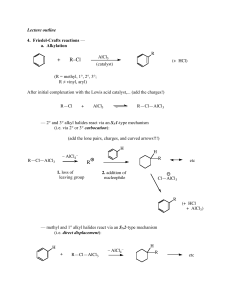
... (hydrazine, base, protic solvent, and lots of heat) avoids the rearrangement problem. (We’ll learn a bit more about these when we get to carbonyl chemistry.) — F-C acylation is quite a general procedure; a variety of different acyl groups can be introduced by this method. Here an a couple of other e ...
... (hydrazine, base, protic solvent, and lots of heat) avoids the rearrangement problem. (We’ll learn a bit more about these when we get to carbonyl chemistry.) — F-C acylation is quite a general procedure; a variety of different acyl groups can be introduced by this method. Here an a couple of other e ...
Basic Chemistry Review
... Polymer decomposition through hydrolysis H2O + polymer monomer + monomer A water molecule is added to break the bond The OH group from the water molecule bonds with the end of one monomer and the H bonds with the end of the other monomer • This breaks the bond between the two monomers ...
... Polymer decomposition through hydrolysis H2O + polymer monomer + monomer A water molecule is added to break the bond The OH group from the water molecule bonds with the end of one monomer and the H bonds with the end of the other monomer • This breaks the bond between the two monomers ...
• Life`s diversity results from the variety of molecules in cells • A
... properties of organic compounds • Functional groups are the groups of atoms that participate in chemical reactions – Hydroxyl groups are characteristic of alcohols – The carboxyl group acts as an acid ...
... properties of organic compounds • Functional groups are the groups of atoms that participate in chemical reactions – Hydroxyl groups are characteristic of alcohols – The carboxyl group acts as an acid ...
Page 1 of 6 NAME __Me___Hulan Jack_______My
... No. The total current through the circuit changes when R3 is removed. But, the current flowing through R1 is always the same current that flows though R2 . The same current flows through all resistors connected in series. b. In the parallel circuit does the total current I change as a result of remo ...
... No. The total current through the circuit changes when R3 is removed. But, the current flowing through R1 is always the same current that flows though R2 . The same current flows through all resistors connected in series. b. In the parallel circuit does the total current I change as a result of remo ...
Name: ______KEY__________________ Date: ______ CHM 130
... 11. (6 pts) The graph to the right represents a temperature versus heat energy plot for a pure substance. Answer the following questions. a. What letter represents pure gas? _E___ b. What phase changes occur at letter B? __melting/freezing___________ c. What is the temperature of the boiling point o ...
... 11. (6 pts) The graph to the right represents a temperature versus heat energy plot for a pure substance. Answer the following questions. a. What letter represents pure gas? _E___ b. What phase changes occur at letter B? __melting/freezing___________ c. What is the temperature of the boiling point o ...
File
... A. Provide the formula for another soluble substance which will form a precipitate when added to a solution of copper (II) chloride. ( 2 pts) B. Write a balanced net ionic equation for the reaction between a solution of the salt you selected in part A, and a solution of copper(II) chloride. ( 3 pts) ...
... A. Provide the formula for another soluble substance which will form a precipitate when added to a solution of copper (II) chloride. ( 2 pts) B. Write a balanced net ionic equation for the reaction between a solution of the salt you selected in part A, and a solution of copper(II) chloride. ( 3 pts) ...
Synthetic Polymers - McQuarrie General Chemistry
... tion reaction, such as the synthesis of nylon, generally involves two different monomers that combine to form the basic repeating unit of the polymer. A polymer made from more than one type of monomer is known as a copolymer, whereas one made from just one type of monomer is called a monopolymer. Ny ...
... tion reaction, such as the synthesis of nylon, generally involves two different monomers that combine to form the basic repeating unit of the polymer. A polymer made from more than one type of monomer is known as a copolymer, whereas one made from just one type of monomer is called a monopolymer. Ny ...
Group Activity 3 [10 PTS]
... 1. Write the condensed structural formula of each of the following alcohols a. 1-propanol ...
... 1. Write the condensed structural formula of each of the following alcohols a. 1-propanol ...
File - ARC: Chemistry
... d. only cations 2. What is the name given to the electrons in the highest occupied energy level of an atom? a. cations c. valence electrons b. anions d. orbital electrons 3. A bond formed between a silicon atom and an oxygen atom is likely to be ____. a. nonpolar covalent c. coordinate covalent b. p ...
... d. only cations 2. What is the name given to the electrons in the highest occupied energy level of an atom? a. cations c. valence electrons b. anions d. orbital electrons 3. A bond formed between a silicon atom and an oxygen atom is likely to be ____. a. nonpolar covalent c. coordinate covalent b. p ...
Intro to Organic Chemistry PPT
... C atoms are versatile building blocks bonding properties 4 stable covalent bonds Bonds with CHNOPS – the elements that make up livingthings ...
... C atoms are versatile building blocks bonding properties 4 stable covalent bonds Bonds with CHNOPS – the elements that make up livingthings ...
Fall 2002 Honors
... 11. (15 pts) What is the pH of a solution produced by dissolving 5.000 g chlorous acid and 5.000 g sodium chlorite in 100.0 mL water? 12. (15 pts) One step in the commercial production of nitric acid is the oxidation of ammonia with oxygen, an exothermic process. The reaction is: NH3 (g) + O2 (g) ! ...
... 11. (15 pts) What is the pH of a solution produced by dissolving 5.000 g chlorous acid and 5.000 g sodium chlorite in 100.0 mL water? 12. (15 pts) One step in the commercial production of nitric acid is the oxidation of ammonia with oxygen, an exothermic process. The reaction is: NH3 (g) + O2 (g) ! ...
Chapter 3 - Biochemistry
... 2. Amino functional group, (NH2), and carboxyl functional group (COOH) 3. amino acids (monomers) Example-Tryptophan a. 20 different kinds 1) all have central carbon bonded to hydrogen 2) R group (radical) - part that differs in each 4. dehyd. syn. joins 2 amino acids to form dipeptide a. OH from aci ...
... 2. Amino functional group, (NH2), and carboxyl functional group (COOH) 3. amino acids (monomers) Example-Tryptophan a. 20 different kinds 1) all have central carbon bonded to hydrogen 2) R group (radical) - part that differs in each 4. dehyd. syn. joins 2 amino acids to form dipeptide a. OH from aci ...
reactions of organic compounds
... • Hydrolysis of an ester produces a carboxylic acid and an alcohol. • The hydrolysis of an amide produces a carboxylic acid and an amine. • Hydrolysis can be acidic or basic hydrolysis. – In acidic: org. Mol. Reacts with water in the presence of an acid. – In basic: org. Mol. Reacts with OH- ion, fr ...
... • Hydrolysis of an ester produces a carboxylic acid and an alcohol. • The hydrolysis of an amide produces a carboxylic acid and an amine. • Hydrolysis can be acidic or basic hydrolysis. – In acidic: org. Mol. Reacts with water in the presence of an acid. – In basic: org. Mol. Reacts with OH- ion, fr ...
Worksheet 1 - Oregon State chemistry
... have a double bond located at the third carbon from the end of the chain. The term omega-3 is used because omega indicates the first carbon at the end of the chain and the 3 indicates the third carbon atom in. ...
... have a double bond located at the third carbon from the end of the chain. The term omega-3 is used because omega indicates the first carbon at the end of the chain and the 3 indicates the third carbon atom in. ...
Chapter 2 Key Terms: element, atom, proton, neutron, electron
... 1. Describe hydrogen bonds and explain how these bonds contribute to each of the five special properties of water. Describe and discuss each of the five properties of water with some detail. 2. Explain what makes a molecule polar or non-polar. Describe what happens to (a) polar and (b) non polar mol ...
... 1. Describe hydrogen bonds and explain how these bonds contribute to each of the five special properties of water. Describe and discuss each of the five properties of water with some detail. 2. Explain what makes a molecule polar or non-polar. Describe what happens to (a) polar and (b) non polar mol ...
Polythiophene

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. They can become conducting when electrons are added or removed from the conjugated π-orbitals via doping. The study of polythiophenes has intensified over the last three decades. The maturation of the field of conducting polymers was confirmed by the awarding of the 2000 Nobel Prize in Chemistry to Alan J. Heeger, Alan MacDiarmid, and Hideki Shirakawa ""for the discovery and development of conductive polymers"". The most notable property of these materials, electrical conductivity, results from the delocalization of electrons along the polymer backbone – hence the term ""synthetic metals"". However, conductivity is not the only interesting property resulting from electron delocalization. The optical properties of these materials respond to environmental stimuli, with dramatic color shifts in response to changes in solvent, temperature, applied potential, and binding to other molecules. Both color changes and conductivity changes are induced by the same mechanism—twisting of the polymer backbone, disrupting conjugation—making conjugated polymers attractive as sensors that can provide a range of optical and electronic responses.A number of comprehensive reviews have been published on PTs, the earliest dating from 1981. Schopf and Koßmehl published a comprehensive review of the literature published between 1990 and 1994. Roncali surveyed electrochemical synthesis in 1992, and the electronic properties of substituted PTs in 1997. McCullough's 1998 review focussed on chemical synthesis of conducting PTs. A general review of conjugated polymers from the 1990s was conducted by Reddinger and Reynolds in 1999. Finally, Swager et al. examined conjugated-polymer-based chemical sensors in 2000. These reviews are an excellent guide to the highlights of the primary PT literature from the last two decades.













![Group Activity 3 [10 PTS]](http://s1.studyres.com/store/data/010780770_1-3445600a9b56e890a0f283c789afe8fb-300x300.png)








