Synthesis of PbS Nanoclusters within Block Copolymer Nanoreactors
... The purified polymer was dried under vacuum for 24 h. The dried polymer was redissolved in THF, and the solution was transferred to a Teflon cup inside a glass jar. The THF was allowed to evaporate slowly over 5-7 days to obtain a transparent polymer film, which was further dried under vacuum for 24 ...
... The purified polymer was dried under vacuum for 24 h. The dried polymer was redissolved in THF, and the solution was transferred to a Teflon cup inside a glass jar. The THF was allowed to evaporate slowly over 5-7 days to obtain a transparent polymer film, which was further dried under vacuum for 24 ...
PowerPoint 프레젠테이션
... Retrosynthetic analysis (or retrosynthesis) : the process of mentally breaking down a molecule into starting materials Retrosynthetic arrow: an open-ended arrow, , used to indicate the reverse of a synthetic reaction Synthon: idealized fragments resulting from a disconnection. Synthons need to be re ...
... Retrosynthetic analysis (or retrosynthesis) : the process of mentally breaking down a molecule into starting materials Retrosynthetic arrow: an open-ended arrow, , used to indicate the reverse of a synthetic reaction Synthon: idealized fragments resulting from a disconnection. Synthons need to be re ...
33 POLYMERS I OPTIONAL MODULE - 2
... like fibres and therefore can be woven into fabrics. The common examples are nylon66, dacron, silk, etc. 3. Thermoplastics : These are linear polymers with very few cross linkages or no cross linkages at all. The polymeric chains are held by weak VANDER WAAL forces and slide over one another. Due to ...
... like fibres and therefore can be woven into fabrics. The common examples are nylon66, dacron, silk, etc. 3. Thermoplastics : These are linear polymers with very few cross linkages or no cross linkages at all. The polymeric chains are held by weak VANDER WAAL forces and slide over one another. Due to ...
Experiment #3: Asymmetric Synthesis – Use of a Chiral Manganese
... Traditionally, olefin epoxidations have been accomplished using a variety of peroxyacids. Since the early 1980's, however, a number of effective transition metal-based catalyst systems have been developed that have significantly extended the scope of these reactions by allowing enantioselective oxyg ...
... Traditionally, olefin epoxidations have been accomplished using a variety of peroxyacids. Since the early 1980's, however, a number of effective transition metal-based catalyst systems have been developed that have significantly extended the scope of these reactions by allowing enantioselective oxyg ...
FREE Sample Here - We can offer most test bank and
... 3. The French chemist Antoine Lavoisier found that the weight of objects before burning and the weight of the products after burning were equal. He concluded that the total weight did not change during a process. Which of these best describes Lavoisier's conclusion? a. From observation, Lavoisier cr ...
... 3. The French chemist Antoine Lavoisier found that the weight of objects before burning and the weight of the products after burning were equal. He concluded that the total weight did not change during a process. Which of these best describes Lavoisier's conclusion? a. From observation, Lavoisier cr ...
Carboxylic Acids - BSAK Chemistry weebly
... A: The polar C=O and the ionic O- group mean that short chain (up to C4) carboxylic acids are readily water soluble due to H bond formation with water molecules. Q: With larger chain acids solubility decreases proportionally with size. Why? A: Hydrocarbon chains are hydrophobic (water fearing) and b ...
... A: The polar C=O and the ionic O- group mean that short chain (up to C4) carboxylic acids are readily water soluble due to H bond formation with water molecules. Q: With larger chain acids solubility decreases proportionally with size. Why? A: Hydrocarbon chains are hydrophobic (water fearing) and b ...
Measurement of Bulk Resistance of Conducting Polymer Films in
... polymers as strain gauge material to measure force, strain or pressure is a recent application that exploits the piezoresistive property of certain conducting polymers [2-4]. Advantages of using conducting polymers as strain gauge materials are high gauge factor and flexibility [5-6] and also compat ...
... polymers as strain gauge material to measure force, strain or pressure is a recent application that exploits the piezoresistive property of certain conducting polymers [2-4]. Advantages of using conducting polymers as strain gauge materials are high gauge factor and flexibility [5-6] and also compat ...
A New Method for Halodecarboxylation of Acids Using Lead(IV
... goes a large shift to lower energies with hydrate formation indicative of strong hydrogen-bond formation, l o and the 0-H stretching band of the water of hydration appears as an intense absorption in the 3100-3300-cm.-’ region with slight anion dependence. Vol’pin, et were dealing with a hydrate; th ...
... goes a large shift to lower energies with hydrate formation indicative of strong hydrogen-bond formation, l o and the 0-H stretching band of the water of hydration appears as an intense absorption in the 3100-3300-cm.-’ region with slight anion dependence. Vol’pin, et were dealing with a hydrate; th ...
Name - marric.us
... ____ 26. As the elements of group 17 are considered in order of increasing atomic number, there is an increase in a. atomic radius c. first ionization energy b. electronegativity d. number of electrons in the first shell ...
... ____ 26. As the elements of group 17 are considered in order of increasing atomic number, there is an increase in a. atomic radius c. first ionization energy b. electronegativity d. number of electrons in the first shell ...
Chapter 23 Functional Groups
... Derivatives of the carboxylic acids, in which the -OH from the carboxyl group is replaced by an -OR from an alcohol: carboxylic acid + alcohol ester + water many ...
... Derivatives of the carboxylic acids, in which the -OH from the carboxyl group is replaced by an -OR from an alcohol: carboxylic acid + alcohol ester + water many ...
Chapter4 - OrgSites.com
... Covalent acids form ions in solution, with the help of the water molecules. For instance, hydrogen chloride molecules, which are polar, give up their hydrogens to water, forming chloride ions (Cl-) and hydronium ions (H3O+). ...
... Covalent acids form ions in solution, with the help of the water molecules. For instance, hydrogen chloride molecules, which are polar, give up their hydrogens to water, forming chloride ions (Cl-) and hydronium ions (H3O+). ...
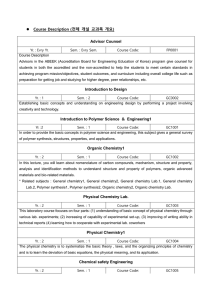
Course Description Word File
... Course Code: GC1022 To increase the design ability of polymer materials by studying the fundamentals of polymer materials and characterization, by selecting polymer materials, which can be found in our living environment and industries, and performing team projects with them in order to learn materi ...
... Course Code: GC1022 To increase the design ability of polymer materials by studying the fundamentals of polymer materials and characterization, by selecting polymer materials, which can be found in our living environment and industries, and performing team projects with them in order to learn materi ...
File
... formula of a member of a homologous series, ie for an alkane: CnH2n+2, (iv) structural formula as the minimal detail that shows the arrangement of atoms in a molecule, eg for butane: CH3CH2CH2CH3 or CH3(CH2)2CH3, (v) displayed formula as the relative positioning of atoms and the bonds between them ( ...
... formula of a member of a homologous series, ie for an alkane: CnH2n+2, (iv) structural formula as the minimal detail that shows the arrangement of atoms in a molecule, eg for butane: CH3CH2CH2CH3 or CH3(CH2)2CH3, (v) displayed formula as the relative positioning of atoms and the bonds between them ( ...
View PDF
... ____ 23. An active metal and a halogen react to form a(n) a. salt. c. acid. b. hydroxide. d. oxide. ____ 24. In the equation 2Al(s) + 3Fe(NO 3 ) 2 (aq) → 3Fe(s) + 2Al(NO 3 ) 3 (aq), iron has been replaced by a. nitrate. c. aluminum. b. water. d. nitrogen. ____ 25. If a certain metal is placed in an ...
... ____ 23. An active metal and a halogen react to form a(n) a. salt. c. acid. b. hydroxide. d. oxide. ____ 24. In the equation 2Al(s) + 3Fe(NO 3 ) 2 (aq) → 3Fe(s) + 2Al(NO 3 ) 3 (aq), iron has been replaced by a. nitrate. c. aluminum. b. water. d. nitrogen. ____ 25. If a certain metal is placed in an ...
Reactions of carbohydrates
... and cytosine produces water and cytodine, one of the structural units of RNA: ...
... and cytosine produces water and cytodine, one of the structural units of RNA: ...
Lecture Resource ()
... The oxidation of a primary alcohol can be stopped at the aldehyde if pyridinium chlorochromate (PCC) is used as the oxidizing agent ...
... The oxidation of a primary alcohol can be stopped at the aldehyde if pyridinium chlorochromate (PCC) is used as the oxidizing agent ...
Chem 3.5 Questions 09
... Compound G is a structural isomer of A with the same functional group. Compare and contrast the reactions of G with the reagents in question (a) to that of compound A. Your answer should include: ...
... Compound G is a structural isomer of A with the same functional group. Compare and contrast the reactions of G with the reagents in question (a) to that of compound A. Your answer should include: ...
Page 1
... Name______________________________________________period______IB chemistry ch. 10 organic chemistry 1. What makes carbon able to form so many different compounds? It bonds to itself to form long chains 2. What is the maximum number of other atoms to which a given carbon atom can be attached? Why? Fo ...
... Name______________________________________________period______IB chemistry ch. 10 organic chemistry 1. What makes carbon able to form so many different compounds? It bonds to itself to form long chains 2. What is the maximum number of other atoms to which a given carbon atom can be attached? Why? Fo ...
Sem-5 - NVPAS
... The infrared spectrum, Infrared spectra of hydrocarbons, Infrared spectra of alcohols, Infrared spectra of ethers, the nuclear magnetic resonance (NMR) spectrum. Number of signals, NMR positions of signals. Chemical shift, NMR peak area and proton counting, NMR Splitting of signals. Spin-spin coupli ...
... The infrared spectrum, Infrared spectra of hydrocarbons, Infrared spectra of alcohols, Infrared spectra of ethers, the nuclear magnetic resonance (NMR) spectrum. Number of signals, NMR positions of signals. Chemical shift, NMR peak area and proton counting, NMR Splitting of signals. Spin-spin coupli ...
Basic overview of the working principle of a
... total of five connectors: WE, CE, RE, S and ground. The potential is always measured between the RE (blue) and the S (red) and the current is always measured between the WE (red) and CE (black). The ground connector (green) can be used to connect external devices to the same ground of the ...
... total of five connectors: WE, CE, RE, S and ground. The potential is always measured between the RE (blue) and the S (red) and the current is always measured between the WE (red) and CE (black). The ground connector (green) can be used to connect external devices to the same ground of the ...
Catalogue of Polymer PTC Resettable Fuse
... Application Automotive applications Automotive harness The conventional solution in wire harnesses is that groups similar circuits together and protects them with a single fuse. In order to limit risk of fire, the wire high current carrying capability, and the oversized wire is commonly used. If ...
... Application Automotive applications Automotive harness The conventional solution in wire harnesses is that groups similar circuits together and protects them with a single fuse. In order to limit risk of fire, the wire high current carrying capability, and the oversized wire is commonly used. If ...
Thiobenzoate Photochemistry
... studies of pyridinethione, 6H-purine-6-thione, and N-hydroxyacridine-9-thione have shown that their excited triplet states are reduced by tetrmethylbenzidine, but no subsequent reactions were reported.16-18 This proposal asks for support to investigate the mechanism, scope and synthetic applications ...
... studies of pyridinethione, 6H-purine-6-thione, and N-hydroxyacridine-9-thione have shown that their excited triplet states are reduced by tetrmethylbenzidine, but no subsequent reactions were reported.16-18 This proposal asks for support to investigate the mechanism, scope and synthetic applications ...
Chapter 23 Functional Groups
... polar, they do not form hydrogen bonds (reason: there is no hydrogen bonded to a highly electronegative atom!) –thus, much lower b.p. than the hydrogen-bonded carboxylic acids they came from ...
... polar, they do not form hydrogen bonds (reason: there is no hydrogen bonded to a highly electronegative atom!) –thus, much lower b.p. than the hydrogen-bonded carboxylic acids they came from ...
Terpyridine-based Materials. For Catalytic, Optoelectronic and Life Science Applications Brochure
... In recent years, the utilization of terpyridines both in macromolecular structure assembly and device chemistry has exploded, enabling, for example, supramolecular polymer architectures with switchable chemical and physical properties as well as novel functional materials for optoelectronic applicat ...
... In recent years, the utilization of terpyridines both in macromolecular structure assembly and device chemistry has exploded, enabling, for example, supramolecular polymer architectures with switchable chemical and physical properties as well as novel functional materials for optoelectronic applicat ...
Polythiophene

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. They can become conducting when electrons are added or removed from the conjugated π-orbitals via doping. The study of polythiophenes has intensified over the last three decades. The maturation of the field of conducting polymers was confirmed by the awarding of the 2000 Nobel Prize in Chemistry to Alan J. Heeger, Alan MacDiarmid, and Hideki Shirakawa ""for the discovery and development of conductive polymers"". The most notable property of these materials, electrical conductivity, results from the delocalization of electrons along the polymer backbone – hence the term ""synthetic metals"". However, conductivity is not the only interesting property resulting from electron delocalization. The optical properties of these materials respond to environmental stimuli, with dramatic color shifts in response to changes in solvent, temperature, applied potential, and binding to other molecules. Both color changes and conductivity changes are induced by the same mechanism—twisting of the polymer backbone, disrupting conjugation—making conjugated polymers attractive as sensors that can provide a range of optical and electronic responses.A number of comprehensive reviews have been published on PTs, the earliest dating from 1981. Schopf and Koßmehl published a comprehensive review of the literature published between 1990 and 1994. Roncali surveyed electrochemical synthesis in 1992, and the electronic properties of substituted PTs in 1997. McCullough's 1998 review focussed on chemical synthesis of conducting PTs. A general review of conjugated polymers from the 1990s was conducted by Reddinger and Reynolds in 1999. Finally, Swager et al. examined conjugated-polymer-based chemical sensors in 2000. These reviews are an excellent guide to the highlights of the primary PT literature from the last two decades.