CH 28 – Atomic Physics
... atoms were like a plum pudding, then the scattering would be more uniform and none would be expected to be completely deflected backwards. Rutherford’s interpretation was that the atom must have a massive, positively charged nucleus. Bohr Theory of the Hydrogen Atom If a tube is filled with a gas su ...
... atoms were like a plum pudding, then the scattering would be more uniform and none would be expected to be completely deflected backwards. Rutherford’s interpretation was that the atom must have a massive, positively charged nucleus. Bohr Theory of the Hydrogen Atom If a tube is filled with a gas su ...
Atomic Physics
... atoms were like a plum pudding, then the scattering would be more uniform and none would be expected to be completely deflected backwards. Rutherford’s interpretation was that the atom must have a massive, positively charged nucleus. Bohr Theory of the Hydrogen Atom If a tube is filled with a gas su ...
... atoms were like a plum pudding, then the scattering would be more uniform and none would be expected to be completely deflected backwards. Rutherford’s interpretation was that the atom must have a massive, positively charged nucleus. Bohr Theory of the Hydrogen Atom If a tube is filled with a gas su ...
A quantum framework for likelihood ratios
... statistical methodology to achieve this. For instance, in the absence of data relating to covariate overlap, the widely used Bayes’ theorem either defaults to the marginal probability driven “naive Bayes’ classifier”, or requires the use of compensatory expectation-maximization techniques. Equally, ...
... statistical methodology to achieve this. For instance, in the absence of data relating to covariate overlap, the widely used Bayes’ theorem either defaults to the marginal probability driven “naive Bayes’ classifier”, or requires the use of compensatory expectation-maximization techniques. Equally, ...
pdf - UMD Physics
... Sketch careful, qualitatively accurate plots for the stated wave functions in each of the potentials shown. Check that your wave function has the correct symmetry, number of nodes, relative wavelengths, maximum values of amplitudes and relative rate of decrease outside the well. (a) The ground state ...
... Sketch careful, qualitatively accurate plots for the stated wave functions in each of the potentials shown. Check that your wave function has the correct symmetry, number of nodes, relative wavelengths, maximum values of amplitudes and relative rate of decrease outside the well. (a) The ground state ...
Electrons!
... Where does this energy come from? Quantum mechanics is a field of physics that answers this. Electrons absorb a specific number of photons of energy when they are excited (heated or absorb some other form of energy). The electrons are not stable in that state and emit photons of energy (in the for ...
... Where does this energy come from? Quantum mechanics is a field of physics that answers this. Electrons absorb a specific number of photons of energy when they are excited (heated or absorb some other form of energy). The electrons are not stable in that state and emit photons of energy (in the for ...
Applied Physics - Full-Time - JNTUH College of Engineering
... The understanding of properties of matter is an essential part to utilize them in various applications in different walks of life. In most of the cases, the behavior of matter as solid material body purely depends upon the internal micro level nature, structure and characters. By studying first few ...
... The understanding of properties of matter is an essential part to utilize them in various applications in different walks of life. In most of the cases, the behavior of matter as solid material body purely depends upon the internal micro level nature, structure and characters. By studying first few ...
Quantum mechanics is the theory that we use to describe the
... Bohr, De Broglie, and others. This theory came to be known as quantum mechanics. ...
... Bohr, De Broglie, and others. This theory came to be known as quantum mechanics. ...
File
... Atomic Structure and Quantum Chemistry Give the one main contribution to the development of the atomic model from each of the following scientists: Dalton, Thomson, Rutherford, Chadwick, and Bohr. (2) Identify elements by both name and chemical symbol using a periodic table. (3) Compare protons, ele ...
... Atomic Structure and Quantum Chemistry Give the one main contribution to the development of the atomic model from each of the following scientists: Dalton, Thomson, Rutherford, Chadwick, and Bohr. (2) Identify elements by both name and chemical symbol using a periodic table. (3) Compare protons, ele ...
Physics 6B Practice midterm 1
... You are designing a hydraulic lift for an automobile garage. It will consist of two oil filled cylindrical pipes of different diameters. A worker pushes down on a piston at one end, raising the car on a platform on the other. To handle a full range of jobs, you must be able to lift cars up to 3000kg ...
... You are designing a hydraulic lift for an automobile garage. It will consist of two oil filled cylindrical pipes of different diameters. A worker pushes down on a piston at one end, raising the car on a platform on the other. To handle a full range of jobs, you must be able to lift cars up to 3000kg ...
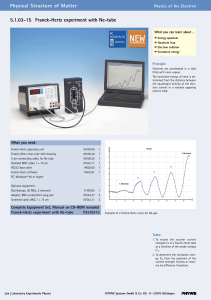
5.1.03-15 Franck-Hertz experiment with Ne
... The electrons emitted by a thermionic cathode are accelerated between cathode C and anode A in the tube filled with neon gas (Fig. 3) and are scattered by elastic collision with neon atoms. From an anode voltage U1 of 16,8 V, however, the kinetic energy of the electrons is sufficient to bring the va ...
... The electrons emitted by a thermionic cathode are accelerated between cathode C and anode A in the tube filled with neon gas (Fig. 3) and are scattered by elastic collision with neon atoms. From an anode voltage U1 of 16,8 V, however, the kinetic energy of the electrons is sufficient to bring the va ...
Chapter 4 - Arrangement of Electrons in Atoms
... 1. Electrons have wavelike properties 2. Consider the electron as a wave confined to a space that can have only certain frequencies B. The Heisenbery Uncertainty Principle (Werner Heisenberg - 1927) 1. "It is impossible to determine simultaneously both the position and velocity of an electron or any ...
... 1. Electrons have wavelike properties 2. Consider the electron as a wave confined to a space that can have only certain frequencies B. The Heisenbery Uncertainty Principle (Werner Heisenberg - 1927) 1. "It is impossible to determine simultaneously both the position and velocity of an electron or any ...
Quantum systems in one-dimension and quantum transport
... IPCMS – Institut de Physique et Chimie des Matériaux de Strasbourg Quantum systems confined to low dimensions, such as spin chains, carbon nanotubes or cold atoms in optical lattices, often behave in a universal way that is efficiently described in terms of simple effective theories. These introduct ...
... IPCMS – Institut de Physique et Chimie des Matériaux de Strasbourg Quantum systems confined to low dimensions, such as spin chains, carbon nanotubes or cold atoms in optical lattices, often behave in a universal way that is efficiently described in terms of simple effective theories. These introduct ...
What is an electron? A century after Bohr conceived of the electron
... different, however. While the strength of the electron's magnetic field provides perhaps the most stringent, and brilliantly successful, comparison of theory and experiment in all of physical science, the value of the electric field has never been measured, and is a mystery even to theory. Establish ...
... different, however. While the strength of the electron's magnetic field provides perhaps the most stringent, and brilliantly successful, comparison of theory and experiment in all of physical science, the value of the electric field has never been measured, and is a mystery even to theory. Establish ...
May 2005
... attached. Just before the current starts to flow, the battery, cable and resistor are all at rest with no external forces. Their total mass is M . After some time, a steady-state current flows along the inner conductor and returns along the outer one. The current may be taken as uniformly distribute ...
... attached. Just before the current starts to flow, the battery, cable and resistor are all at rest with no external forces. Their total mass is M . After some time, a steady-state current flows along the inner conductor and returns along the outer one. The current may be taken as uniformly distribute ...
The Interaction of Radiation and Matter: Quantum Theory
... very complex and much studied matter. The simple interpretation of Equation [ V-38a ] is problematic since the integrand is proportional to at large and, thus, the correction significantly diverges!! The divergence in was for many years an unresolved discrepancy between the quantum theory of radiati ...
... very complex and much studied matter. The simple interpretation of Equation [ V-38a ] is problematic since the integrand is proportional to at large and, thus, the correction significantly diverges!! The divergence in was for many years an unresolved discrepancy between the quantum theory of radiati ...
Chap8_theatom
... Three quantum numbers determine the size and shape of the probability cloud of an atomic electron: Quantum Number – (n) is the chief factor that governs the electron’s energy. Orbital Quantum Number – (l) determines the magnitude of the electron’s angular momentum. Magnetic Quantum Number – (ml) ...
... Three quantum numbers determine the size and shape of the probability cloud of an atomic electron: Quantum Number – (n) is the chief factor that governs the electron’s energy. Orbital Quantum Number – (l) determines the magnitude of the electron’s angular momentum. Magnetic Quantum Number – (ml) ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.