ps700-coll2-hayden
... double slit experiment we want to know the positions on a screen at which the electrons arrive individually, one at a time. Over time, the positions at which the electrons are detected build up a pattern characteristic of wave interference. The usual Copenhagen interpretation is puzzling in that a ...
... double slit experiment we want to know the positions on a screen at which the electrons arrive individually, one at a time. Over time, the positions at which the electrons are detected build up a pattern characteristic of wave interference. The usual Copenhagen interpretation is puzzling in that a ...
Entanglement and Bell theorem
... • A source must emit pairs of discrete-state systems, which can be detected with high efficiency. • QM must predict strong correlations of the relevant observables of each pair, and the pairs must have high QM purity. • Analyzers must have extremely high fidelity to allow transmittance of desired st ...
... • A source must emit pairs of discrete-state systems, which can be detected with high efficiency. • QM must predict strong correlations of the relevant observables of each pair, and the pairs must have high QM purity. • Analyzers must have extremely high fidelity to allow transmittance of desired st ...
ppt - ICTS
... entropy (roughly 0.1n) yet we can recover all of x prefectly with probability 10% We therefore have to use the fact that the dimension of the encoding is low (2n/8) ...
... entropy (roughly 0.1n) yet we can recover all of x prefectly with probability 10% We therefore have to use the fact that the dimension of the encoding is low (2n/8) ...
CHAPTER 3: The Experimental Basis of Quantum Theory
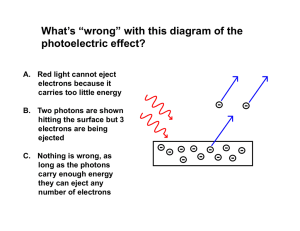
... Classical theory predicts that the total amount of energy in a light wave increases as the light intensity increases. The maximum kinetic energy of the photoelectrons depends on the value of the light frequency f and not on the intensity. The existence of a threshold frequency is completely inexplic ...
... Classical theory predicts that the total amount of energy in a light wave increases as the light intensity increases. The maximum kinetic energy of the photoelectrons depends on the value of the light frequency f and not on the intensity. The existence of a threshold frequency is completely inexplic ...
Electron Spin I - Rutgers Physics
... • The electron is an elementary (point-like) particle. It has three intrinsic properties: mass, electric charge and angular momentum (spin). It is hard to grasp how a point particle with no size and therefore no moment of inertia can have an angular momentum. Nevertheless, the electron carries intri ...
... • The electron is an elementary (point-like) particle. It has three intrinsic properties: mass, electric charge and angular momentum (spin). It is hard to grasp how a point particle with no size and therefore no moment of inertia can have an angular momentum. Nevertheless, the electron carries intri ...
Electrons in Atoms
... include sub-energy levels. Consequently, shells are seperated into subshells each of which is represented with angular momentum quantum number “l” .This determines the geometrical shape of the electron probability distribution. The number “l” can have all values ranging from 0, 1, 2 to n-1. For n=1 ...
... include sub-energy levels. Consequently, shells are seperated into subshells each of which is represented with angular momentum quantum number “l” .This determines the geometrical shape of the electron probability distribution. The number “l” can have all values ranging from 0, 1, 2 to n-1. For n=1 ...
on one possibility of making a medium transparent by
... presented by us in [2J, where it was shown that the conditions (14) are realized automatically under conditions of strict resonance at frequency 2w and in the presence of spatial synchronism, and such a state of the interacting concrete waves is stable. As to the effect of suppression of two-photon ...
... presented by us in [2J, where it was shown that the conditions (14) are realized automatically under conditions of strict resonance at frequency 2w and in the presence of spatial synchronism, and such a state of the interacting concrete waves is stable. As to the effect of suppression of two-photon ...
7-0838-fassihi
... close slits. By this Feynman concluded that we can never say from which slit the photon passes and therefore can never be localised. What is missing in his observation is that the photon we observe in the interference pattern is a secondary photon and is not the original. Here in fact we have no dir ...
... close slits. By this Feynman concluded that we can never say from which slit the photon passes and therefore can never be localised. What is missing in his observation is that the photon we observe in the interference pattern is a secondary photon and is not the original. Here in fact we have no dir ...
Objective Test (2) on Quantum Numbers MM: 30 Time : 45 min
... Match the correct enthalpy with the elements and complete the graph given in Fig. 3.1. Also write symbols of elements with their atomic number. ...
... Match the correct enthalpy with the elements and complete the graph given in Fig. 3.1. Also write symbols of elements with their atomic number. ...
Chapter 27
... principle: If a measurement of position of a particle is made with precision Δx and a simultaneous measurement of linear momentum is made with precision Δpx, then the product of the two uncertainties can never be smaller than h/4 ...
... principle: If a measurement of position of a particle is made with precision Δx and a simultaneous measurement of linear momentum is made with precision Δpx, then the product of the two uncertainties can never be smaller than h/4 ...
energy - Edublogs
... The electrons, in their orbitals about the nucleus, have QUANTIZED levels of energy that are determined by which orbital they are in. The orbitals are numbered with “n” numbers, the “principle quantum number”: n = 1, n = 2, n = 3, etc. where the orbital closest to the nucleus is n = 1. The “n-numbe ...
... The electrons, in their orbitals about the nucleus, have QUANTIZED levels of energy that are determined by which orbital they are in. The orbitals are numbered with “n” numbers, the “principle quantum number”: n = 1, n = 2, n = 3, etc. where the orbital closest to the nucleus is n = 1. The “n-numbe ...
a Multicromophoric approach to describe the energy
... master equation doesn’t use this approximation, but it takes a longer time to be computed numerically. Our simulations compared with the exact result show that not always the Secular approximation is correct and off diagonal terms are needed. The picture shows this test both for room and cryogenic t ...
... master equation doesn’t use this approximation, but it takes a longer time to be computed numerically. Our simulations compared with the exact result show that not always the Secular approximation is correct and off diagonal terms are needed. The picture shows this test both for room and cryogenic t ...
Document
... Complex atoms contain more than one electron, so the interaction between electrons must be accounted for in the energy levels. This means that the energy depends on both n and . A neutral atom has Z electrons, as well as Z protons in its nucleus. Z is called the atomic number. ...
... Complex atoms contain more than one electron, so the interaction between electrons must be accounted for in the energy levels. This means that the energy depends on both n and . A neutral atom has Z electrons, as well as Z protons in its nucleus. Z is called the atomic number. ...
Lecture 1 Atomic Structure
... A: n = 3 is the number of the shell. It can have l = 0, 1, and 2 l = 2 means that these are the d orbitals. For l = 2, there are five values of ml (-2, -1, 0, +1, +2) So, the all five orbitals below are the correct answer to this question. (In the exam, giving just one answer is ok.) 3dxy, 3dxz, 3dy ...
... A: n = 3 is the number of the shell. It can have l = 0, 1, and 2 l = 2 means that these are the d orbitals. For l = 2, there are five values of ml (-2, -1, 0, +1, +2) So, the all five orbitals below are the correct answer to this question. (In the exam, giving just one answer is ok.) 3dxy, 3dxz, 3dy ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.