AP Chemistry-midterm review
... ____ 51. Heating MgSO4•7H2O at 150 C produces MgSO4•xH2O. If heating 24.4 g of pure MgSO4•7H2O at 150 C were to give 13.7 g of pure MgSO4•xH2O, calculate the value for x. a. 5 b. 4 c. 3 d. 2 e. 1 ____ 52. An ore of lead is 45.0% pure lead sulfide, PbS, and 55.0% impurities in which no other lead com ...
... ____ 51. Heating MgSO4•7H2O at 150 C produces MgSO4•xH2O. If heating 24.4 g of pure MgSO4•7H2O at 150 C were to give 13.7 g of pure MgSO4•xH2O, calculate the value for x. a. 5 b. 4 c. 3 d. 2 e. 1 ____ 52. An ore of lead is 45.0% pure lead sulfide, PbS, and 55.0% impurities in which no other lead com ...
Chapter 3
... We noted in Section 2.2 that with a plentiful supply of oxygen, carbon burns to form carbon dioxide. With a limited supply of oxygen, carbon monoxide is formed. How can we find out what minimum quantity of oxygen is needed to ensure that when carbon burns, it is completely converted to carbon dioxid ...
... We noted in Section 2.2 that with a plentiful supply of oxygen, carbon burns to form carbon dioxide. With a limited supply of oxygen, carbon monoxide is formed. How can we find out what minimum quantity of oxygen is needed to ensure that when carbon burns, it is completely converted to carbon dioxid ...
Chapter 3 Solutions - Bremerton School District
... When balancing reactions, start with elements that appear in only one of the reactants and one of the products, then go on to balance the remaining elements. a. Fe + O2 ÷ Fe2O3. Balancing Fe first, then O, gives: 2 Fe + 3/2 O2 ÷ Fe2O3. The best balanced equation contains the smallest whole numbers. ...
... When balancing reactions, start with elements that appear in only one of the reactants and one of the products, then go on to balance the remaining elements. a. Fe + O2 ÷ Fe2O3. Balancing Fe first, then O, gives: 2 Fe + 3/2 O2 ÷ Fe2O3. The best balanced equation contains the smallest whole numbers. ...
Solutions for Chapter 8 End-of-Chapter Problems
... (a) Ice cream melting will decrease the organization of the system. Rather than being in identifiable scoops of ice cream perched securely on a cone, there now will be liquid ice cream dripping on the outside of the cone, onto your hands, and possibly onto your clothes as well. (b) Students entering ...
... (a) Ice cream melting will decrease the organization of the system. Rather than being in identifiable scoops of ice cream perched securely on a cone, there now will be liquid ice cream dripping on the outside of the cone, onto your hands, and possibly onto your clothes as well. (b) Students entering ...
Study Guide Chapter 10: An Introduction to Chemistry
... is an equation stoichiometry problem. 7. For some chemical reactions, chemists want to mix reactants in amounts that are as close as possible to the ratio that would lead to the complete reaction of each. This ratio is sometimes called the stoichiometric ratio. 9. Sometimes one product is more impor ...
... is an equation stoichiometry problem. 7. For some chemical reactions, chemists want to mix reactants in amounts that are as close as possible to the ratio that would lead to the complete reaction of each. This ratio is sometimes called the stoichiometric ratio. 9. Sometimes one product is more impor ...
Stoichiometry
... Moles, mass, representative particles (atoms, molecules, formula units), molar mass, and Avogadro’s number. The percent composition of an element in a compound. Balanced chemical equations: for example, for a given mass of a reactant, calculate the amount of produced. Limiting reactants: calcula ...
... Moles, mass, representative particles (atoms, molecules, formula units), molar mass, and Avogadro’s number. The percent composition of an element in a compound. Balanced chemical equations: for example, for a given mass of a reactant, calculate the amount of produced. Limiting reactants: calcula ...
Topic 1 Quantitative Chemistry Answers - slider-dpchemistry-11
... A substance that cannot be divided into simpler, smaller substances. In an element, all the atoms have the same number of protons or electrons, but the number of neutrons may vary (more about this Topic 2) b) atom The smallest part of an element that can exist. An atom consists of an extremely tiny ...
... A substance that cannot be divided into simpler, smaller substances. In an element, all the atoms have the same number of protons or electrons, but the number of neutrons may vary (more about this Topic 2) b) atom The smallest part of an element that can exist. An atom consists of an extremely tiny ...
CHEMISTRY CHM-050 Introduction to Chemistry I NCC Cr: 3 D Lec
... Lec Y Lab: Y Prerequisite: MAT-063, Elementary Algebra, or equivalent. A onesemester college chemistry course which surveys important concepts and topics of chemistry. Among these are the metric system of measurement, atomic theory of matter, energy levels and atomic structure, the periodic table, i ...
... Lec Y Lab: Y Prerequisite: MAT-063, Elementary Algebra, or equivalent. A onesemester college chemistry course which surveys important concepts and topics of chemistry. Among these are the metric system of measurement, atomic theory of matter, energy levels and atomic structure, the periodic table, i ...
chapter 2 - chemical equations and reaction yields
... CHAPTER 2 - CHEMICAL EQUATIONS AND REACTION YIELDS ...
... CHAPTER 2 - CHEMICAL EQUATIONS AND REACTION YIELDS ...
Soln Chem 2008Nov(9746)
... that of chemically pure N2, the gas that causes this discrepancy would, therefore, be one of higher mass than N2. [Mr : N2 = 28; Ar = 39.9; He = 4; CH4 = 16; Ne = 20.0] (ans) ...
... that of chemically pure N2, the gas that causes this discrepancy would, therefore, be one of higher mass than N2. [Mr : N2 = 28; Ar = 39.9; He = 4; CH4 = 16; Ne = 20.0] (ans) ...
Chapter 3: Mass Relationships in Chemical
... Chapter 3: Mass Relationships in Chemical Reactions A periodic table will be required to answer some of these questions. 1. An atom of helium has a mass about four times greater than that of an atom of hydrogen. Which choice makes the correct comparison of the relative numbers of helium and hydrogen ...
... Chapter 3: Mass Relationships in Chemical Reactions A periodic table will be required to answer some of these questions. 1. An atom of helium has a mass about four times greater than that of an atom of hydrogen. Which choice makes the correct comparison of the relative numbers of helium and hydrogen ...
Problem 1-2
... An additional fifth component of waterless air varies strongly depending on season, position and height. On the one hand it protects life on earth, on the other hand it leads to damages indirect exposition. b) ...
... An additional fifth component of waterless air varies strongly depending on season, position and height. On the one hand it protects life on earth, on the other hand it leads to damages indirect exposition. b) ...
Solutions Manual
... © Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006. This page from Chemistry Contexts 2 Teacher’s Resource second edition may be reproduced for classroom use. ...
... © Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006. This page from Chemistry Contexts 2 Teacher’s Resource second edition may be reproduced for classroom use. ...
Proposed syllabus and Scheme of Examination B.Sc. (Program) with
... and angular nodes and their significance. Radial distribution functions and the concept of the most probable distance with special reference to 1s and 2s atomic orbitals. Significance of quantum numbers, orbital angular momentum and quantum numbers ml and ms. Shapes of s, p and d atomic orbitals, no ...
... and angular nodes and their significance. Radial distribution functions and the concept of the most probable distance with special reference to 1s and 2s atomic orbitals. Significance of quantum numbers, orbital angular momentum and quantum numbers ml and ms. Shapes of s, p and d atomic orbitals, no ...
CHAPTER 9
... A balanced chemical equation is the key step in all stoichiometric calculations, because the mole ratio is obtained directly from it. Solving any reaction stoichiometry problem must begin with a balanced equation. Chemical equations help us plan the amounts of reactants to use in a chemical reaction ...
... A balanced chemical equation is the key step in all stoichiometric calculations, because the mole ratio is obtained directly from it. Solving any reaction stoichiometry problem must begin with a balanced equation. Chemical equations help us plan the amounts of reactants to use in a chemical reaction ...
Chemistry Science Notebook: Student Edition
... to take notes, use graphic organizers, learn vocabulary, and develop their thinking skills by writing in order to achieve academic success. The ability to take and organize notes predicts how well students will do in school. Peverly, Brobst, Graham, and Shaw (2003) showed that when students use back ...
... to take notes, use graphic organizers, learn vocabulary, and develop their thinking skills by writing in order to achieve academic success. The ability to take and organize notes predicts how well students will do in school. Peverly, Brobst, Graham, and Shaw (2003) showed that when students use back ...
Stoichiometry - Normal Community High School Chemistry
... Moles, mass, representative particles (atoms, molecules, formula units), molar mass, and Avogadro’s number. The percent composition of an element in a compound. Balanced chemical equations: for example, for a given mass of a reactant, calculate the amount of produced. Limiting reactants: calcula ...
... Moles, mass, representative particles (atoms, molecules, formula units), molar mass, and Avogadro’s number. The percent composition of an element in a compound. Balanced chemical equations: for example, for a given mass of a reactant, calculate the amount of produced. Limiting reactants: calcula ...
App. Chemistry
... Interpretation of electronic spectra including charge transfer spectra, spectrochemical series, nephelauxetic series, metal clusters, sandwich compounds, metal carbonyls b) Bioinorganic Chemistry 05h Role of metal ions in biological processes, structure and properties of metalloproteins in electron ...
... Interpretation of electronic spectra including charge transfer spectra, spectrochemical series, nephelauxetic series, metal clusters, sandwich compounds, metal carbonyls b) Bioinorganic Chemistry 05h Role of metal ions in biological processes, structure and properties of metalloproteins in electron ...
Thermochemistry - hrsbstaff.ednet.ns.ca
... body, which is about 37˚C. You can feel the difference as warmth when you drink the hot chocolate, as shown in Figure 16.4. As you drink, the particles in the hot chocolate transfer kinetic energy as heat to particles in your mouth. The particles in the hot chocolate slow down, and the particles in ...
... body, which is about 37˚C. You can feel the difference as warmth when you drink the hot chocolate, as shown in Figure 16.4. As you drink, the particles in the hot chocolate transfer kinetic energy as heat to particles in your mouth. The particles in the hot chocolate slow down, and the particles in ...
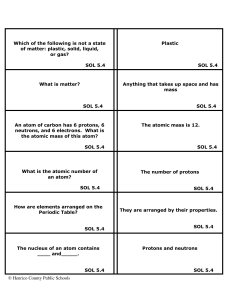
Matter Flashcards 5 - Henrico County Public Schools
... make it up. For instance, Sodium (Na) is an explosive substance and Chlorine (Cl) is a poisonous gas, but combined together, they make NaCl, or table salt, which is not poisonous or explosive. SOL 5.4 ...
... make it up. For instance, Sodium (Na) is an explosive substance and Chlorine (Cl) is a poisonous gas, but combined together, they make NaCl, or table salt, which is not poisonous or explosive. SOL 5.4 ...
Chemistry
... Gaseous state: Kinetic molecular model of a gas: postulates and derivation of the kinetic gas equation; collision frequency; collision diameter; mean free path and viscosity of gases, including their temperature and pressure dependence, relation between mean free path and coefficient of viscosity, c ...
... Gaseous state: Kinetic molecular model of a gas: postulates and derivation of the kinetic gas equation; collision frequency; collision diameter; mean free path and viscosity of gases, including their temperature and pressure dependence, relation between mean free path and coefficient of viscosity, c ...
Cyclam ``capa` POT.4` to ``capa` POT.3` denticity change
... decided, therefore, to study Ru complexes with mono-Nsubstituted cyclam ligands containing an arm bearing amine or carboxy functional groups. These groups are versatile linkers because they can form amide bonds with a desired material or relevant biomolecules such as proteins or antibodies. Whereas ...
... decided, therefore, to study Ru complexes with mono-Nsubstituted cyclam ligands containing an arm bearing amine or carboxy functional groups. These groups are versatile linkers because they can form amide bonds with a desired material or relevant biomolecules such as proteins or antibodies. Whereas ...