12_chemistry_impq_CH13_amines_02
... C2 H5NH2 +CH3COCl ---------Æ C2H5NHCOCH3+ HCl Q9.Why are amines basic in character? ANS. Like ammonia, the nitrogen atom in amines RNH2 is trivalent and bears an unshared pair of electrons. Thus it acts like a Lewis base and donates the pair of electrons to electrondeficient species which further in ...
... C2 H5NH2 +CH3COCl ---------Æ C2H5NHCOCH3+ HCl Q9.Why are amines basic in character? ANS. Like ammonia, the nitrogen atom in amines RNH2 is trivalent and bears an unshared pair of electrons. Thus it acts like a Lewis base and donates the pair of electrons to electrondeficient species which further in ...
Mark scheme F325 Equilibria, Energetics and Elements June
... DO NOT ALLOW responses using nuclear size or attraction DO NOT ALLOW responses linked with loss of electrons IGNORE larger electron density ALLOW smaller sum of radii gives a greater ionic attraction IGNORE NaBr has greater ionic attraction IGNORE NaBr has smallest ionic radius (not focussing on siz ...
... DO NOT ALLOW responses using nuclear size or attraction DO NOT ALLOW responses linked with loss of electrons IGNORE larger electron density ALLOW smaller sum of radii gives a greater ionic attraction IGNORE NaBr has greater ionic attraction IGNORE NaBr has smallest ionic radius (not focussing on siz ...
Massachusetts Tests for Educator Licensure (MTEL )
... Correct Response: B. The environmental conditions are controlled, and the comparison stated is correct and sufficient to answer the question as to which plastic degrades more quickly. A is incorrect because the two plastics are not compared and degradation rates are theoretical. C is incorrect becau ...
... Correct Response: B. The environmental conditions are controlled, and the comparison stated is correct and sufficient to answer the question as to which plastic degrades more quickly. A is incorrect because the two plastics are not compared and degradation rates are theoretical. C is incorrect becau ...
Chapter 6: Thermochemistry
... kJ/mol, whereas the standard enthalpy of formation of sodium carbonate monohydrate is –1430.1 kJ/mol. Determine H° at 25°C for the reaction Na2CO3(s) + H2O(l) Na2CO3·H2O(s). (Given: H°f[H2O(l)] = –285.8 kJ/mol) A) –13.4 kJ/mol D) –299.2 kJ/mol B) –285.8 kJ/mol E) –156.3 kJ/mol C) –585.0 kJ/mol A ...
... kJ/mol, whereas the standard enthalpy of formation of sodium carbonate monohydrate is –1430.1 kJ/mol. Determine H° at 25°C for the reaction Na2CO3(s) + H2O(l) Na2CO3·H2O(s). (Given: H°f[H2O(l)] = –285.8 kJ/mol) A) –13.4 kJ/mol D) –299.2 kJ/mol B) –285.8 kJ/mol E) –156.3 kJ/mol C) –585.0 kJ/mol A ...
Chapter 4
... Strategy: Hydrogen displacement: Any metal above hydrogen in the activity series will displace it from water or from an acid. Metals below hydrogen will not react with either water or an acid. Solution: Only (b) Li and (d) Ca are above hydrogen in the activity series, so they are the only metals in ...
... Strategy: Hydrogen displacement: Any metal above hydrogen in the activity series will displace it from water or from an acid. Metals below hydrogen will not react with either water or an acid. Solution: Only (b) Li and (d) Ca are above hydrogen in the activity series, so they are the only metals in ...
Unit 4 - Chemical Equilibrium
... When the _ _ _ _ _ in a chemical reaction looks like this ⇌ , it shows that the reaction is _ _ _ _ _ _ _ _ _ _. This means the products can react together and turn back into the _ _ _ _ _ _ _ _ reactants. In other words, the reaction can go _ _ _ _ ways. When a reversible reaction is set up in a _ ...
... When the _ _ _ _ _ in a chemical reaction looks like this ⇌ , it shows that the reaction is _ _ _ _ _ _ _ _ _ _. This means the products can react together and turn back into the _ _ _ _ _ _ _ _ reactants. In other words, the reaction can go _ _ _ _ ways. When a reversible reaction is set up in a _ ...
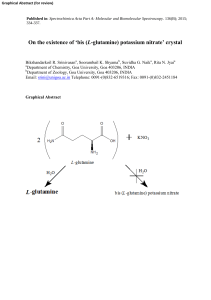
`bis (L-glutamine) potassium nitrate` crystal
... 2). The grown crystals exhibit a positive optical rotation confirming them to be same as those of the starting material namely L(+)-glutamine. The optical activity data indicate that use of alkali metal nitrate does not result in any isomerisation / racemisation of the amino acid. The present study ...
... 2). The grown crystals exhibit a positive optical rotation confirming them to be same as those of the starting material namely L(+)-glutamine. The optical activity data indicate that use of alkali metal nitrate does not result in any isomerisation / racemisation of the amino acid. The present study ...
6 Chemical Bonding – Orbital Theory
... valence orbital on one atom ‘overlaps’ the electron cloud of the other bonding atom to form a covalent linkage. On the contrary, the electrovalent bond formation involves a physical transfer of the electron and the orbital concept is not very useful for their explanation. The theory of ‘maximum over ...
... valence orbital on one atom ‘overlaps’ the electron cloud of the other bonding atom to form a covalent linkage. On the contrary, the electrovalent bond formation involves a physical transfer of the electron and the orbital concept is not very useful for their explanation. The theory of ‘maximum over ...
Review of N and Metal co-Doped TiO for Water Purification under
... towards larger visible spectra, which is partially due to reducing the band gap of the semiconductor by N doping. Ag nanoparticles could transfer the plasmonic energy from the Ag0 to the TiO2 semiconductor due to its surface plasmonic resonance (SPR) effect [40], this also contributed to enlarging l ...
... towards larger visible spectra, which is partially due to reducing the band gap of the semiconductor by N doping. Ag nanoparticles could transfer the plasmonic energy from the Ag0 to the TiO2 semiconductor due to its surface plasmonic resonance (SPR) effect [40], this also contributed to enlarging l ...
File
... determining ΔG oreaction is: ΔG° = Σnp Δnof (products) Σnr ΔGof (reactants) . Because ΔG is a state function (path independent), chemical reactions with known ΔG values can be manipulated to determine ΔG for a different reaction. ΔG for the different reaction is the sum of ΔG for all the step ...
... determining ΔG oreaction is: ΔG° = Σnp Δnof (products) Σnr ΔGof (reactants) . Because ΔG is a state function (path independent), chemical reactions with known ΔG values can be manipulated to determine ΔG for a different reaction. ΔG for the different reaction is the sum of ΔG for all the step ...
C H A P T E R
... Atoms, ions, and molecules are very small, so even tiny samples have a huge number of particles. To make counting such large numbers easier, scientists use the same approach to represent the number of ions or molecules in a sample as they use for atoms. The SI unit for amount is called the mole (mol ...
... Atoms, ions, and molecules are very small, so even tiny samples have a huge number of particles. To make counting such large numbers easier, scientists use the same approach to represent the number of ions or molecules in a sample as they use for atoms. The SI unit for amount is called the mole (mol ...
quantitative chemistry
... acidity). The properties of the mixture are similar to those of the components (e.g. a match burns in both air and pure oxygen), though they will vary with its exact composition. The fact that the different components of the mixture have different physical properties means that the mixture can be se ...
... acidity). The properties of the mixture are similar to those of the components (e.g. a match burns in both air and pure oxygen), though they will vary with its exact composition. The fact that the different components of the mixture have different physical properties means that the mixture can be se ...
Problem 1-2
... There is no carbon in the unknown substance A. If 19.5 g of A is annealed in the absence of air a ternary, white, crystalline compound B and a gas C form. In the presence of air gas C burns with a light blue flame. The elementary analysis of B shows 24.5 % (w/w) of carbon and 28.6 % (w/w) of nitroge ...
... There is no carbon in the unknown substance A. If 19.5 g of A is annealed in the absence of air a ternary, white, crystalline compound B and a gas C form. In the presence of air gas C burns with a light blue flame. The elementary analysis of B shows 24.5 % (w/w) of carbon and 28.6 % (w/w) of nitroge ...
CHAPTER SIXTEEN SPONTANEITY, ENTROPY, AND FREE
... determining ΔG oreaction is: ΔG° = Σnp Δn of (products) Σnr ΔG of (reactants) . Because ΔG is a state function (path independent), chemical reactions with known ΔG values can be manipulated to determine ΔG for a different reaction. ΔG for the different reaction is the sum of ΔG for all the st ...
... determining ΔG oreaction is: ΔG° = Σnp Δn of (products) Σnr ΔG of (reactants) . Because ΔG is a state function (path independent), chemical reactions with known ΔG values can be manipulated to determine ΔG for a different reaction. ΔG for the different reaction is the sum of ΔG for all the st ...
Study Guide for Content Mastery - Student Edition
... makes up the lowest level. This layer is called the (2) . This level contains a protective ...
... makes up the lowest level. This layer is called the (2) . This level contains a protective ...
Problem 28. TUNNELING IN CHEMISTRY
... in detail the properties of three unknown elements that were ekaboron (Eb), ekaaluminum (Ea), and ekasilicon (Es). All of them were discovered in the next 15 years. ...
... in detail the properties of three unknown elements that were ekaboron (Eb), ekaaluminum (Ea), and ekasilicon (Es). All of them were discovered in the next 15 years. ...
Chapter 4 Classifying Reactions: Chemicals in Balance
... Synthesis reactions follow the general pattern, A + B → C. Add each pair of reactants and write the chemical formula of the expected product. Balance these synthesis reactions by a back-and-forth process of reasoning, as was used in Practice Problems 5 and 6. Act on Your Strategy (a) Fe + O2 → 2 Fe ...
... Synthesis reactions follow the general pattern, A + B → C. Add each pair of reactants and write the chemical formula of the expected product. Balance these synthesis reactions by a back-and-forth process of reasoning, as was used in Practice Problems 5 and 6. Act on Your Strategy (a) Fe + O2 → 2 Fe ...
FREE Sample Here
... 28) The smallest particle of an element that retains the characteristics of the element is a(n) ________. A) electron B) neutron C) proton D) atom E) nucleus Answer: D Page Ref: 4.3 Learning Obj.: 4.3 Global Outcomes: G7 Demonstrate the ability to make connections between concepts across chemistry. ...
... 28) The smallest particle of an element that retains the characteristics of the element is a(n) ________. A) electron B) neutron C) proton D) atom E) nucleus Answer: D Page Ref: 4.3 Learning Obj.: 4.3 Global Outcomes: G7 Demonstrate the ability to make connections between concepts across chemistry. ...
chapter 20 - United International College
... The species that can oxidize water to molecular oxygen must have an Ered more positive than 1.23 V. From Table 19.1 of the text we see that only Cl2(g) and MnO4(aq) in acid solution can oxidize water to oxygen. ...
... The species that can oxidize water to molecular oxygen must have an Ered more positive than 1.23 V. From Table 19.1 of the text we see that only Cl2(g) and MnO4(aq) in acid solution can oxidize water to oxygen. ...
National German competition
... To become a member of the German IChO-team you have to be successful in four rounds. The problems to be solved in the 1st round are sent to all highschools. To solve the problems the students may use all resources available, e.g. textbooks etc. All the students who solve about 70% will receive the p ...
... To become a member of the German IChO-team you have to be successful in four rounds. The problems to be solved in the 1st round are sent to all highschools. To solve the problems the students may use all resources available, e.g. textbooks etc. All the students who solve about 70% will receive the p ...
Stoichiometry - Social Circle City Schools
... As you learned in Unit 1, atoms are so small and have such small masses that any amount of atoms we would work with would be very hard to count. For example, a piece of aluminum about the size of a pencil eraser contains approximately 2 × 1022 aluminum atoms! The mole (abbreviated mol) is the unit c ...
... As you learned in Unit 1, atoms are so small and have such small masses that any amount of atoms we would work with would be very hard to count. For example, a piece of aluminum about the size of a pencil eraser contains approximately 2 × 1022 aluminum atoms! The mole (abbreviated mol) is the unit c ...
Physical Chemistry 3: — Chemical Kinetics
... The scriptum gives a summary of the material covered in the scheduled lectures to allow students to repeat the material more economically. It covers basic material that all chemistry students should learn irrespective of their possible inclination towards inorganic, organic or physical chemistry, bu ...
... The scriptum gives a summary of the material covered in the scheduled lectures to allow students to repeat the material more economically. It covers basic material that all chemistry students should learn irrespective of their possible inclination towards inorganic, organic or physical chemistry, bu ...